Reversible Disorder-to-Order Transition Induced by Aqueous Lithiation in Vanadate Electrode Materials
IF 7.2
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
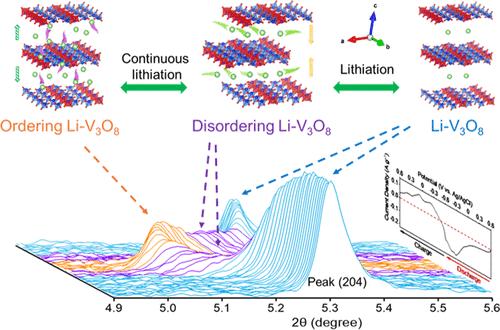
Vanadium-based oxides are intriguing electrode materials in aqueous electrochemical systems owing to their low cost and high theoretical capacity for alkali storage, especially lithium (Li) ions. However, a sequence of phase transformations and irreversible structure distortion upon Li-ion intercalation causes structural instability and has been a lingering problem for vanadium oxide electrodes. Here, we investigate lithium vanadate (Li–V3O8) for aqueous Li-ion intercalation and deintercalation processes. Unlike its crystalline V2O5 polymorph, Li–V3O8 retains monophasic lithiation, which is attributed to its disordered crystalline nature and large interplanar distance. Importantly, we show a unique and reversible sequence of disorder-to-order structural transition induced by the extent of lithiation, which indicates sequential interlayer and intralayer lithiation process, and vice versa in delithiation process, supported by electrokinetic analysis, in situ X-ray diffraction (XRD), and Debye scattering simulations. The absence of distortive phase transitions and multilithiation pathways facilitates Li-ion diffusion across the vanadate electrode materials to improve storage capacity. This work opens a new dimension for vanadium-based disordered oxides, accelerating the development of low-cost, aqueous electrochemical systems.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemistry of Materials
工程技术-材料科学:综合
CiteScore
14.10
自引率
5.80%
发文量
929
审稿时长
1.5 months
期刊介绍:
The journal Chemistry of Materials focuses on publishing original research at the intersection of materials science and chemistry. The studies published in the journal involve chemistry as a prominent component and explore topics such as the design, synthesis, characterization, processing, understanding, and application of functional or potentially functional materials. The journal covers various areas of interest, including inorganic and organic solid-state chemistry, nanomaterials, biomaterials, thin films and polymers, and composite/hybrid materials. The journal particularly seeks papers that highlight the creation or development of innovative materials with novel optical, electrical, magnetic, catalytic, or mechanical properties. It is essential that manuscripts on these topics have a primary focus on the chemistry of materials and represent a significant advancement compared to prior research. Before external reviews are sought, submitted manuscripts undergo a review process by a minimum of two editors to ensure their appropriateness for the journal and the presence of sufficient evidence of a significant advance that will be of broad interest to the materials chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


