Safe and Sustainable Industrial-Scale Production of Anhydrous Diazomethane via a Fully DCS/SIS-Controlled Continuous Flow System: Synthesis of α-Haloketones
IF 3.1
3区 化学
Q2 CHEMISTRY, APPLIED
引用次数: 0
Abstract
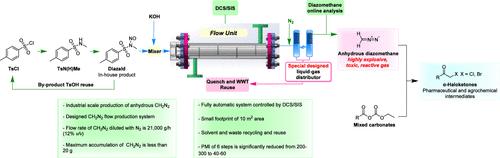
Anhydrous diazomethane in nitrogen (12% v/v, moisture at <0.5 g/m3) has been safely manufactured via a fully automation controlled continuous microchannel reactor on a 1500 kg per month scale while the maximum accumulation of diazomethane was less than 20 g. Treatment of N-methyl-N-nitroso-p-toluenesulfonamide (MNTS, diazald) with KOH generated diazomethane in situ, which was isolated by a custom-designed gas–liquid distributor. Subsequently, the afforded anhydrous diazomethane was used to synthesize α-haloketones, which serve as key intermediates for anti-HIV and antihypertensive drugs such as Atazanavir and Nebivolol. The entire process from TsCl to α-haloketones had been continuously operated at the ABAChem manufacturing site for more than 6 months, affording a total of 5000 kg of pure diazomethane and 15,000 kg of α-haloketones smoothly without any safety issues. The microchannel flow reactor and gas–liquid distributor were designed specifically for the process and equipped with a fully distributed control system and safety instrumented system (DCS/SIS) to ensure safety, quality, and efficiency. The wastewater and solvent had been recycled and reused, while the process mass intensity for the six chemical synthesis steps from TsCl to the corresponding α-haloketones was significantly reduced from 200 to 300 to 40–60. This production protocol paves a safer, sustainable, and expedient access to industrial-scale synthesis of α-haloketones and other intermediates useful for production of pharmaceutical and agrochemical substances involving diazomethane.
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
6.90
自引率
14.70%
发文量
251
审稿时长
2 months
期刊介绍:
The journal Organic Process Research & Development serves as a communication tool between industrial chemists and chemists working in universities and research institutes. As such, it reports original work from the broad field of industrial process chemistry but also presents academic results that are relevant, or potentially relevant, to industrial applications. Process chemistry is the science that enables the safe, environmentally benign and ultimately economical manufacturing of organic compounds that are required in larger amounts to help address the needs of society. Consequently, the Journal encompasses every aspect of organic chemistry, including all aspects of catalysis, synthetic methodology development and synthetic strategy exploration, but also includes aspects from analytical and solid-state chemistry and chemical engineering, such as work-up tools,process safety, or flow-chemistry. The goal of development and optimization of chemical reactions and processes is their transfer to a larger scale; original work describing such studies and the actual implementation on scale is highly relevant to the journal. However, studies on new developments from either industry, research institutes or academia that have not yet been demonstrated on scale, but where an industrial utility can be expected and where the study has addressed important prerequisites for a scale-up and has given confidence into the reliability and practicality of the chemistry, also serve the mission of OPR&D as a communication tool between the different contributors to the field.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


