Maleimide-Dependent Rh(III)-Catalyzed Site-Selective Mono and Dual C–H Functionalization of 2-Arylbenzo[d]thiazole and Oxazole Derivatives
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
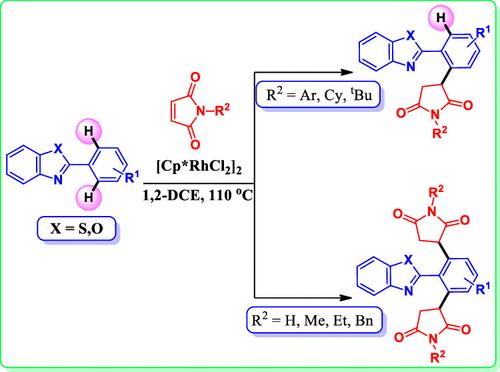
The site-selective functionalization of aromatic compounds via C–H activation has emerged as a popular tool in organic synthesis. In this study, we report a regioselective coupling of maleimide to 2-arylbenzo[d]thiazoles in the presence of a rhodium(III) catalyst. Depending upon the nature of the substituent (R2-group) present in the maleimide substrate, either mono- or bis-1,4-addition products were observed in this methodology. In the case of R2 = aryl, cyclohexyl, and tert-butyl, mono coupling was observed, whereas substituents, such as methyl, ethyl, benzyl, and methyl thiophene, provided bis coupling as the major products. Similar selectivity was also observed in the case of 2-arylbenzo[d]oxazoles.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


