Heterotypic Interactions of Amyloid β and the Islet Amyloid Polypeptide Produce Mixed Aggregates with Non-Native Fibril Structure
IF 4.8
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
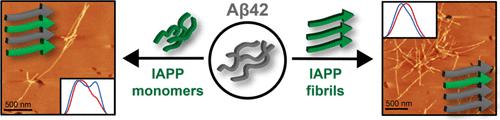
Amyloid aggregates are hallmarks of the pathology of a wide range of diseases, including type 2 diabetes (T2D) and Alzheimer’s disease (AD). Much epidemiological and pathological evidence points to significant overlap between AD and T2D. Individuals with T2D have a higher likelihood of developing AD; moreover, colocalized aggregates of amyloid β (Aβ) and the islet amyloid polypeptide (IAPP), the two main peptides implicated in the formation of toxic amyloid aggregates in AD and T2D, have also been identified in the brain. However, how these peptides interact with each other is not well understood, and the structural facets of heterotypic mixed fibrils formed via such interactions remain elusive. Here we use atomic force microscopy augmented with infrared spectroscopy to probe the secondary structure of individual aggregates formed via heterotypic interactions of Aβ and IAPP and provide unequivocal direct evidence of mixed aggregates. Furthermore, we show that co-aggregation of the peptides from the monomeric stage leads to the formation of unique polymorphs, in which both peptides undergo structural deviation from their native states, whereas seeding with preformed IAPP fibrils leads to aggregates similar to native Aβ. These findings highlight how heterotypic interactions between amyloidogenic peptides can lead to polymorphic diversity proteinopathies.
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Physical Chemistry Letters
CHEMISTRY, PHYSICAL-NANOSCIENCE & NANOTECHNOLOGY
CiteScore
9.60
自引率
7.00%
发文量
1519
审稿时长
1.6 months
期刊介绍:
The Journal of Physical Chemistry (JPC) Letters is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, chemical physicists, physicists, material scientists, and engineers. An important criterion for acceptance is that the paper reports a significant scientific advance and/or physical insight such that rapid publication is essential. Two issues of JPC Letters are published each month.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


