A copper(II)-based metal–organic framework: electrochemical sensing of Cd(II) and Pb(II) and adsorption of organic dyes
IF 3.5
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
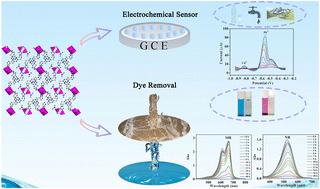
A new inorganic–organic hybrid complex, namely [Cu3L2(DMF)2]·H2O (Cu-L), has been synthesized using a sulfur-rich ligand, 3,3′,3′′-(1,3,5-triazine-2,4,6-triyltrisulfanediyl)tripropanoic acid (H3L) and metal cations under hydrothermal conditions. The metal atoms are interconnected to form a paddle-wheel-like structure, which is ultimately linked to the ligands to create a three-dimensional architecture. Cu-L, employed to fabricate an electrochemical sensor denoted as Cu-L@GCE (glassy carbon electrode), is capable of simultaneously detecting Cd2+ and Pb2+ at approximately −0.82 V and −0.58 V (vs. Ag/AgCl reference), exhibiting high sensitivity and selectivity. Cu-L@GCE demonstrates broad linear detection ranges of 8–28 μM for Cd2+ and 2–14 μM for Pb2+, along with low limit of detection (LOD) values of 0.01363 μM and 0.00212 μM, respectively. Furthermore, Cu-L@GCE achieves LOD values of 0.00209 μM and 0.000034 μM when detecting both ions simultaneously. The constructed sensor successfully detects Cd2+ and Pb2+ in mineral water, tap water, and river water with satisfactory recoveries. Additionally, the adsorption performance for organic dyes has been studied in detail using Cu-L as an adsorbent. The results indicate good adsorption selectivity for methylene blue (MB) and neutral red (NR) compared to methyl orange (MO) and rhodamine B (RhB) molecules.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Dalton Transactions
化学-无机化学与核化学
CiteScore
6.60
自引率
7.50%
发文量
1832
审稿时长
1.5 months
期刊介绍:
Dalton Transactions is a journal for all areas of inorganic chemistry, which encompasses the organometallic, bioinorganic and materials chemistry of the elements, with applications including synthesis, catalysis, energy conversion/storage, electrical devices and medicine. Dalton Transactions welcomes high-quality, original submissions in all of these areas and more, where the advancement of knowledge in inorganic chemistry is significant.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


