Biomimetic Dendritic Cell-Based Nanovaccines for Reprogramming the Immune Microenvironment to Boost Tumor Immunotherapy
IF 16
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Although dendritic cell (DC)-mediated immunotherapies are effective options for immunotherapy, traditional DC vaccines are hampered by a variety of drawbacks such as insufficient antigen delivery, weak lymph node homing, and the risk of living cell transfusion. To address the above-mentioned issues, we developed a personalized DC-mimicking nanovaccine (HybridDC) that enhances antigen presentation and elicits effective antitumor immunity. The biomimetic nanovaccine contains cell membranes derived from genetically engineered DCs, and several cellular components are simultaneously anchored onto these membranes, including CC-chemokine receptor 7 (CCR7), tumor-associated antigenic (TAA) peptide/tumor-derived exosome (TEX), and relevant costimulatory molecules. Compared with previous vaccines, the HybridDC vaccine showed an increased ability to target lymphoid tissues and reshape the immune landscape in the tumor milieu. HybridDC demonstrated significant therapeutic and prophylactic efficacy in poorly immunogenic, orthotopic models of glioma. Furthermore, the HybridDC vaccine potentiates the therapeutic efficacy of immune checkpoint blockade (ICB) therapy, providing a potential combination strategy to maximize the efficacy of ICB. Specifically, HybridDC can induce long-term protective immunity in memory T cells. Overall, the HybridDC vaccine is a promising platform for personalized cancer vaccines and may offer a combinational modality to improve current immunotherapy.
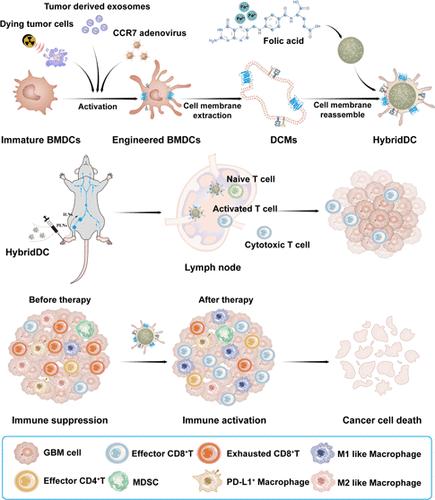
基于仿生树突状细胞的纳米疫苗用于免疫微环境重编程以促进肿瘤免疫治疗
尽管树突状细胞(DC)介导的免疫疗法是免疫治疗的有效选择,但传统的DC疫苗受到各种缺陷的阻碍,如抗原递送不足、淋巴结归巢弱以及活细胞输血的风险。为了解决上述问题,我们开发了一种个性化的dc模拟纳米疫苗(HybridDC),增强抗原呈递并引发有效的抗肿瘤免疫。这种仿生纳米疫苗含有从基因工程树突状细胞中提取的细胞膜,并且几种细胞成分同时固定在这些细胞膜上,包括cc趋化因子受体7 (CCR7)、肿瘤相关抗原(TAA)肽/肿瘤衍生外泌体(TEX)和相关的共刺激分子。与以前的疫苗相比,HybridDC疫苗显示出靶向淋巴组织和重塑肿瘤环境免疫景观的能力增强。HybridDC在免疫原性差的原位胶质瘤模型中显示出显著的治疗和预防效果。此外,HybridDC疫苗增强了免疫检查点阻断(ICB)疗法的治疗效果,提供了一种潜在的联合策略来最大化ICB的疗效。具体来说,HybridDC可以在记忆性T细胞中诱导长期保护性免疫。总的来说,HybridDC疫苗是一个很有前途的个性化癌症疫苗平台,可能提供一种组合方式来改善当前的免疫治疗。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


