Parallel gut-to-brain pathways orchestrate feeding behaviors
IF 21.2
1区 医学
Q1 NEUROSCIENCES
引用次数: 0
Abstract
The caudal nucleus of the solitary tract (cNTS) in the brainstem serves as a hub for integrating interoceptive cues from diverse sensory pathways. However, the mechanisms by which cNTS neurons transform these signals into behaviors remain debated. We analyzed 18 cNTS-Cre mouse lines and cataloged the dynamics of nine cNTS cell types during feeding. We show that Th+ cNTS neurons encode esophageal mechanical distension and transient gulp size via vagal afferent inputs, providing quick feedback regulation of ingestion speed. By contrast, Gcg+ cNTS neurons monitor intestinal nutrients and cumulative ingested calories and have long-term effects on food satiation and preference. These nutritive signals are conveyed through a portal vein–spinal ascending pathway rather than vagal sensory neurons. Our findings underscore distinctions among cNTS subtypes marked by differences in temporal dynamics, sensory modalities, associated visceral organs and ascending sensory pathways, all of which contribute to specific functions in coordinated feeding regulation. This study identifies parallel gut–brain pathways that fine-tune feeding. Distinct brainstem neurons and visceral afferents either sense esophageal stretch to regulate eating speed or detect gut nutrients to shape long-term food choices and satiety.

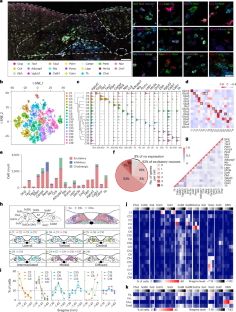
平行的肠-脑通路协调进食行为
脑干孤立束(cNTS)的尾侧核是整合来自不同感觉通路的内感受性信号的中枢。然而,碳纳米管神经元将这些信号转化为行为的机制仍存在争议。我们分析了18个cNTS- cre小鼠品系,并记录了9种cNTS细胞类型在喂养过程中的动态变化。我们发现Th+ cNTS神经元通过迷走神经传入输入编码食道机械膨胀和短暂吞下量,提供摄食速度的快速反馈调节。相比之下,Gcg+ cNTS神经元监测肠道营养物质和累积摄入的卡路里,并对食物饱腹感和偏好有长期影响。这些营养信号是通过门静脉-脊髓上行通路而不是迷走感觉神经元传递的。我们的研究结果强调了cNTS亚型之间的差异,其特征是时间动态、感觉方式、相关内脏器官和上行感觉通路的差异,所有这些都有助于协调摄食调节的特定功能。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature neuroscience
医学-神经科学
CiteScore
38.60
自引率
1.20%
发文量
212
审稿时长
1 months
期刊介绍:
Nature Neuroscience, a multidisciplinary journal, publishes papers of the utmost quality and significance across all realms of neuroscience. The editors welcome contributions spanning molecular, cellular, systems, and cognitive neuroscience, along with psychophysics, computational modeling, and nervous system disorders. While no area is off-limits, studies offering fundamental insights into nervous system function receive priority.
The journal offers high visibility to both readers and authors, fostering interdisciplinary communication and accessibility to a broad audience. It maintains high standards of copy editing and production, rigorous peer review, rapid publication, and operates independently from academic societies and other vested interests.
In addition to primary research, Nature Neuroscience features news and views, reviews, editorials, commentaries, perspectives, book reviews, and correspondence, aiming to serve as the voice of the global neuroscience community.
文献相关原料
公司名称
产品信息
索莱宝
Fast Green
索莱宝
0.02 mg ml?1 Fast Green
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


