Visible-light-promoted synthesis of alkylated Indolo[2,1-α]isoquinolines using 2‑Mercaptothiazolinium salts as alkyl radical source
IF 1.5
4区 化学
Q3 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
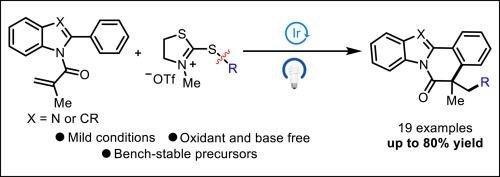
A visible-light-induced radical cascade cyclization of 2-aryl-N-acryloyl indoles with 2‑mercaptothiazolinium salts is reported, furnishing the corresponding indolo[2,1-α]isoquinoline derivatives in good to moderate yields. A series of primary, secondary, and tertiary alkyl bromides were converted conveniently into redox-active thiazolinium salts, which are subsequently transformed into alkyl radicals through interaction with an excited-state photocatalyst. This green and environmentally protocol features broad substrate scope under mild conditions.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Tetrahedron Letters
化学-有机化学
CiteScore
3.50
自引率
5.60%
发文量
521
审稿时长
28 days
期刊介绍:
Tetrahedron Letters provides maximum dissemination of outstanding developments in organic chemistry. The journal is published weekly and covers developments in techniques, structures, methods and conclusions in experimental and theoretical organic chemistry. Rapid publication of timely and significant research results enables researchers from all over the world to transmit quickly their new contributions to large, international audiences.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


