Three-dimensional nanocomposites derived from combined metal organic frameworks doped with Ce, La, and Cu as a bifunctional electrocatalyst for supercapacitors and oxygen reduction reaction
IF 8.1
2区 工程技术
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Fabrication of a nanocomposite with a low-cost and efficient synthesis method instead of using electrocatalysts based on platinum metal has become one of the main challenges in energy storage devices and fuel cells. In this regard, a bifunctional electrocatalyst for supercapacitors and oxygen reduction reaction is fabricated and tested. The novelty of this study is the synthesis method and enhancement of the electrochemical characteristics of synthesized electrocatalysts. The core-shell method is used for the electrocatalyst's synthesis which uses Zeolitic Imidazolate Framework (ZIF)-8, ZIF-67, and Material Institute Lavoisiers (MIL)-101 for the fabrication of three types of electrocatalysts. In the following, to increase the characteristics such as conductivity and stability, doping of Copper (Cu), Cerium (Ce), and Lanthanum (La) are added to the nanocomposites. The Co@NC, CoZn@NC, and CoZn@FeNC prepared electrocatalysts are obtained from the pyrolyze process of La/ZIF-67, CeCu/ZIF-8@ La/ZIF-67, MIL-101@CeCu/ZIF-8/La/ZIF-67. The results indicated that the CoZn@NC electrocatalyst has the best performance in the oxygen reduction reaction (ORR) with an onset potential of 0.062 (V vs Ag/AgCl) and current density of −12.97 (mA/cm2) at a constant voltage of −1 V. Furthermore, the electron transfer number of CoZn@NC electrocatalyst for ORR was 3.64. The conducted Galvanostatic Charge-Discharge (GCD) tests demonstrated that the CoZn@NC electrode has the highest capacitance of 271.14 F/g. The outcomes showed that by the core-shell method, various properties of nanocomposites can be utilized to solve the weaknesses of catalysts by using proper metals. Moreover, the presence of improve the structural defects in the carbon matrix, the stability based on the chronoamperometry results, and the mass transfer. The study provides a perspective for future researches to fill the research gaps to obtain the new supercapacitors utilize on a large scale in the electronics industry.
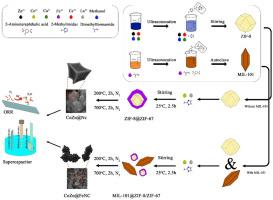
三维纳米复合材料是由掺有Ce、La和Cu的复合金属有机框架制成的,可作为超级电容器和氧还原反应的双功能电催化剂
利用低成本、高效率的合成方法制备纳米复合材料而不是使用基于铂金属的电催化剂,已成为储能装置和燃料电池的主要挑战之一。为此,制备并测试了一种用于超级电容器和氧还原反应的双功能电催化剂。本研究的新颖之处在于所合成电催化剂的合成方法和电化学特性的提高。采用核壳法合成了三种类型的电催化剂,其中ZIF -8、ZIF-67和材料研究所拉瓦锡(MIL)-101分别制备了三种类型的电催化剂。接下来,为了提高纳米复合材料的导电性和稳定性,我们在纳米复合材料中添加了铜(Cu)、铈(Ce)和镧(La)。通过La/ZIF-67、CeCu/ZIF-8@ La/ZIF-67、MIL-101@CeCu/ZIF-8/La/ZIF-67热解制得Co@NC、CoZn@NC和CoZn@FeNC电催化剂。结果表明,CoZn@NC电催化剂在−1 V恒定电压下,起始电位为0.062 (V vs Ag/AgCl),电流密度为−12.97 (mA/cm2),在氧还原反应(ORR)中表现最佳。此外,CoZn@NC电催化剂对ORR的电子转移数为3.64。进行的恒流充放电(GCD)测试表明,CoZn@NC电极的最高电容为271.14 F/g。结果表明,通过核壳方法,可以利用纳米复合材料的各种性能,通过适当的金属来解决催化剂的弱点。此外,Cu2+、Ce3+、La3+的存在改善了碳基体的结构缺陷,提高了碳基体的稳定性和传质性能。该研究为未来的研究提供了一个视角,以填补研究空白,使新型超级电容器在电子工业中大规模应用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

International Journal of Hydrogen Energy
工程技术-环境科学
CiteScore
13.50
自引率
25.00%
发文量
3502
审稿时长
60 days
期刊介绍:
The objective of the International Journal of Hydrogen Energy is to facilitate the exchange of new ideas, technological advancements, and research findings in the field of Hydrogen Energy among scientists and engineers worldwide. This journal showcases original research, both analytical and experimental, covering various aspects of Hydrogen Energy. These include production, storage, transmission, utilization, enabling technologies, environmental impact, economic considerations, and global perspectives on hydrogen and its carriers such as NH3, CH4, alcohols, etc.
The utilization aspect encompasses various methods such as thermochemical (combustion), photochemical, electrochemical (fuel cells), and nuclear conversion of hydrogen, hydrogen isotopes, and hydrogen carriers into thermal, mechanical, and electrical energies. The applications of these energies can be found in transportation (including aerospace), industrial, commercial, and residential sectors.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


