Fe-Mg alloy nitrogen carrier for chemical looping ammonia synthesis process formed by mechanochemical nitrogen fixation and heating hydrogenation
IF 6.7
1区 工程技术
Q2 ENERGY & FUELS
引用次数: 0
Abstract
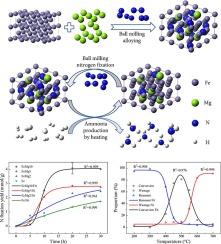
Ammonia is an ideal hydrogen storage material and can be directly used as a carbon-free energy substance. At the same time, ammonia is widely used in producing fertilizers, ensuring global food supply. The demand for ammonia will continue to increase in the future. However, the Haber-Bosch process for ammonia production has problems with high energy consumption and high carbon emission, which is no longer adapted to the requirements of developing a low-carbon and environmentally friendly society. In this paper, a two-step process for ammonia synthesis by ball milling nitrogen fixation and heating was developed by mechanical alloying and chemical looping methods. Ball milling of iron powder for ammonia synthesis proved feasible but less efficient. Under the same conditions, the amount of nitrogen fixed by adding 10 wt% magnesium is about three times that of pure iron powder. After nitrogen fixation, ammonia was produced by heating in an argon-hydrogen mixture. After 1 h of reaction, the nitrogen conversion rate reached about 50 %, and the final nitrogen conversion rate exceeded 80 %. After ammonia release, the carrier can be directly ball-milled for nitrogen fixation to form a chemical looping cycle. Magnesium changes the grain size, lattice size, and microstrain of iron and also transfers some electrons to iron, so it plays a dual role in structural and electronic additives. This process still has great potential for optimization and is easily coupled with renewable energy, which is expected to compete with industrial ammonia synthesis in the future.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Fuel
工程技术-工程:化工
CiteScore
12.80
自引率
20.30%
发文量
3506
审稿时长
64 days
期刊介绍:
The exploration of energy sources remains a critical matter of study. For the past nine decades, fuel has consistently held the forefront in primary research efforts within the field of energy science. This area of investigation encompasses a wide range of subjects, with a particular emphasis on emerging concerns like environmental factors and pollution.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


