An injectable in situ-forming hydrogel with self-activating genipin-chitosan (GpCS) cross-linking and an O2/Ca2+ self-supplying capability for wound healing and rapid hemostasis
IF 10.7
1区 化学
Q1 CHEMISTRY, APPLIED
引用次数: 0
Abstract
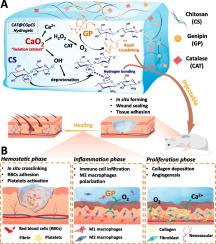
Severe traumatic bleeding and chronic diabetic wounds require rapid hemostasis and multifunctional dressings, which remain particularly challenging, especially for non-compressible trauma and irregular wounds with dysregulated microenvironments. Chitosan (CS) can be easily cross-linked with genipin to form GpCS hydrogels. However, developing injectable GpCS hydrogels for biomedical applications faces challenges, particularly in enhancing rapid gel formation and optimizing physical properties. In this study, we present an innovative approach to improve these aspects by designing a novel injectable GpCS hydrogel, strategically enhanced through a calcium peroxide (CaO2)-activated cross-linking reaction. CaO2 played a pivotal role in promoting in situ cross-linking of the GpCS hydrogel, leading to significant improvements in its injectable in situ gel-forming ability, mechanical strength, and self-healing and bioadhesive properties. CaO2 incorporated in the hydrogels rapidly converted to oxygen when combined with catalase (CAT), thereby establishing a self-sustaining oxygen/calcium release system. This system not only promoted hyperoxia and activated the coagulation cascade, facilitating rapid blood clotting, but also significantly accelerated wound healing through enhanced angiogenesis, collagen deposition, and M2 macrophage polarization. These attributes significantly enhanced the capacity of the hydrogel to facilitate wound closure and hemostasis, highlighting its therapeutic value in accelerating recovery and improving healing outcomes in clinical wound care.
一种具有自激活吉尼平-壳聚糖(GpCS)交联和O2/Ca2+自供能力的可注射性原位形成水凝胶,用于伤口愈合和快速止血
严重创伤性出血和慢性糖尿病性伤口需要快速止血和多功能敷料,这仍然是特别具有挑战性的,特别是对于不可压缩的创伤和微环境失调的不规则伤口。壳聚糖(CS)可以很容易地与genipin交联形成GpCS水凝胶。然而,开发用于生物医学应用的可注射GpCS水凝胶面临着挑战,特别是在增强快速凝胶形成和优化物理性质方面。在这项研究中,我们提出了一种创新的方法来改善这些方面,通过设计一种新型的可注射GpCS水凝胶,通过过氧化钙(CaO2)激活的交联反应战略性地增强。CaO2在促进GpCS水凝胶的原位交联中发挥了关键作用,显著提高了GpCS水凝胶的注射原位成胶能力、机械强度、自愈和生物粘附性能。加入水凝胶中的CaO2与过氧化氢酶(CAT)结合后迅速转化为氧气,从而建立了一个自我维持的氧/钙释放系统。该系统不仅促进高氧,激活凝血级联,促进血液快速凝固,而且通过增强血管生成、胶原沉积和M2巨噬细胞极化,显著加速创面愈合。这些特性显著增强了水凝胶促进伤口关闭和止血的能力,突出了其在加速伤口恢复和改善临床伤口护理愈合效果方面的治疗价值。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Carbohydrate Polymers
化学-高分子科学
CiteScore
22.40
自引率
8.00%
发文量
1286
审稿时长
47 days
期刊介绍:
Carbohydrate Polymers stands as a prominent journal in the glycoscience field, dedicated to exploring and harnessing the potential of polysaccharides with applications spanning bioenergy, bioplastics, biomaterials, biorefining, chemistry, drug delivery, food, health, nanotechnology, packaging, paper, pharmaceuticals, medicine, oil recovery, textiles, tissue engineering, wood, and various aspects of glycoscience.
The journal emphasizes the central role of well-characterized carbohydrate polymers, highlighting their significance as the primary focus rather than a peripheral topic. Each paper must prominently feature at least one named carbohydrate polymer, evident in both citation and title, with a commitment to innovative research that advances scientific knowledge.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


