Amorphous CuSbOx composite-catalyzed electrocatalytic reduction of CO2 to CO: CO2 demand-supply-regulated performance
IF 19.1
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
The path to practical production of targeted chemicals and fuels application via carbon dioxide reduction reactions (CO2RRs) remains a significant challenge mainly due to low CO2 solubility. Aiming to tackle this key issue, herein, we used the CuSbOx cathode-catalyzed reduction of CO2 to CO as a model system to quantitatively depict CO2 demand-supply and performance relationships. We propose a cathode/electrolyte interface model consisting of a porous catalyst layer, and we combined the experimental and computational COMSOL Multiphysics finite-element studies to quantitatively unveil CO2 demand-supply relationships and determine the maximum CO2 supply capacity in both stationary H cell and gas diffusion electrode (GDE) flow cell. This work exemplifies that experimentally measured catalytic performance may not accurately reflect the maximum capacity/intrinsic electrocatalytic activity of electrocatalysts and reveals that CO2 supply capacity in the GDE flow cell can be dramatically affected by the thickness of the liquid layer between the hydrophobic gas diffusion layer and the catalyst layer.
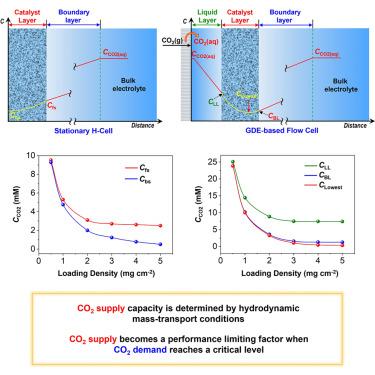
非晶CuSbOx复合材料催化电催化CO2还原为CO: CO2供需调节性能
由于二氧化碳溶解度低,通过二氧化碳还原反应(CO2RRs)实现目标化学品的实际生产和燃料的应用仍然是一个重大挑战。为了解决这一关键问题,本文采用CuSbOx阴极催化CO2还原为CO作为模型系统,定量描述CO2供需和性能关系。我们提出了一个由多孔催化剂层组成的阴极/电解质界面模型,并将实验和计算COMSOL多物理场有限元研究相结合,定量揭示了二氧化碳的供需关系,并确定了固定氢电池和气体扩散电极(GDE)流动电池的最大二氧化碳供应能力。这项工作表明,实验测量的催化性能可能不能准确地反映电催化剂的最大容量/固有电催化活性,并揭示了GDE流动电池中的CO2供应能力会受到疏水气体扩散层和催化剂层之间液体层的厚度的显著影响。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chem
Environmental Science-Environmental Chemistry
CiteScore
32.40
自引率
1.30%
发文量
281
期刊介绍:
Chem, affiliated with Cell as its sister journal, serves as a platform for groundbreaking research and illustrates how fundamental inquiries in chemistry and its related fields can contribute to addressing future global challenges. It was established in 2016, and is currently edited by Robert Eagling.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


