Co-Generation of Hydrogen and FDCA from Biomass-Based HMF in a 3D-Printed Flow Electrolyzer
IF 3.8
3区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
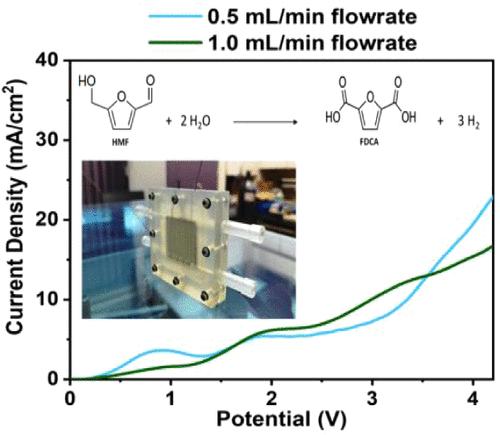
Among all the available resources, biomass is the key renewable resource to capture carbon dioxide from the atmosphere and produce fuels, chemicals, and other value-added products. This work uses an electrochemical process to generate value-added chemicals and hydrogen simultaneously from a biomass-derived platform chemical. A 3D-printed flow electrolyzer is used to study the generation of hydrogen and FDCA (2,5-furandicarboxylic acid) from HMF (5-(hydroxymethyl)furan-2-carbaldehyde) using an alkaline electrolyte based on the principles of electrochemical oxidation. A 3D-printed electrolytic cell is designed with a channel size of 55 mm × 55 mm × 6 mm and an electrocatalyst area of 6.25 cm2 in the form of an anode and cathode. In this work, gold-sputtered nickel foam is used as an anode, while platinum-sputtered nickel foam is used as a cathode. A single pass through the electrolyzer yields 130 μmol/(h cm2) of hydrogen gas at ambient temperature and pressure, along with 46 μmol/(h cm2) of FDCA. A maximum value of 80% conversion of HMF is obtained at a flow rate of 0.5 mL/min in a single pass with a potential bias of 3.5 V. This work opens the pathways for incorporating a microflow electrolyzer to coproduce FDCA and hydrogen from biomass-derived HMF.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Industrial & Engineering Chemistry Research
工程技术-工程:化工
CiteScore
7.40
自引率
7.10%
发文量
1467
审稿时长
2.8 months
期刊介绍:
ndustrial & Engineering Chemistry, with variations in title and format, has been published since 1909 by the American Chemical Society. Industrial & Engineering Chemistry Research is a weekly publication that reports industrial and academic research in the broad fields of applied chemistry and chemical engineering with special focus on fundamentals, processes, and products.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


