Electrochemical formation of bis(fluorosulfonyl)imide-derived solid-electrolyte interphase at Li-metal potential
IF 20.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Lithium bis(fluorosulfonyl)imide-based liquid electrolytes are promising for realizing high coulombic efficiency and long cycle life in next-generation Li-metal batteries. However, the role of anions in the formation of the solid–electrolyte interphase remains unclear. Here we combine electrochemical analyses and X-ray photoelectron spectroscopy measurements, both with and without sample washing, together with computational simulations, to propose the reaction pathways of electrolyte decomposition and correlate the interphase component solubility with the efficacy of passivation. We discover that not all the products derived from interphase-forming reactions are incorporated into the resulting passivation layer, with a notable portion present in the liquid electrolyte. We also find that the high-performance electrolytes can afford a sufficiently passivating interphase with minimized electrolyte decomposition, by incorporating more anion-decomposition products. Overall, this work presents a systematic approach of coupling electrochemical and surface analyses to paint a comprehensive picture of solid–electrolyte interphase formation, while identifying the key attributes of high-performance electrolytes to guide future designs. Li-metal batteries often utilize liquid electrolytes that yield a solid–electrolyte interphase on electrodes; however, the role of anions in interphase formation remains unclear. Now it has been shown that anion-decomposition products provide varying contributions to interphase formation and that high-performance electrolytes balance effective interfacial passivation with minimized degradation.

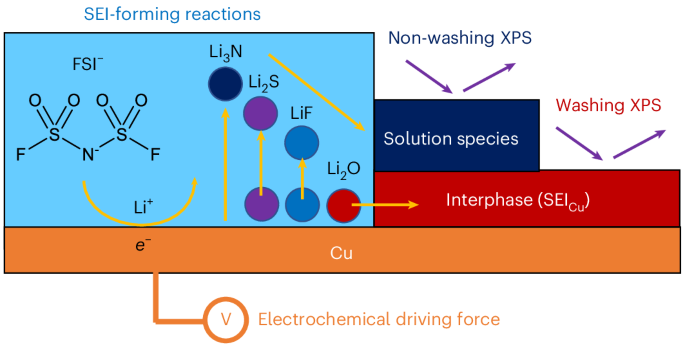
锂金属电位下双(氟磺酰基)亚胺衍生固体-电解质间相的电化学形成
氟磺酰亚胺锂基液体电解质有望在下一代锂金属电池中实现高库仑效率和长循环寿命。然而,阴离子在固-电解质间相形成中的作用尚不清楚。在这里,我们结合电化学分析和x射线光电子能谱测量,在有和没有样品洗涤的情况下,结合计算模拟,提出了电解质分解的反应途径,并将相间组分的溶解度与钝化效果联系起来。我们发现,并非所有形成相间反应的产物都被纳入到所产生的钝化层中,其中很大一部分存在于液体电解质中。我们还发现,通过加入更多的阴离子分解产物,高性能电解质可以提供充分钝化的界面相,使电解质分解最小化。总的来说,这项工作提出了一种耦合电化学和表面分析的系统方法,以描绘固体电解质界面形成的全面图景,同时确定高性能电解质的关键属性,以指导未来的设计。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature chemistry
化学-化学综合
CiteScore
29.60
自引率
1.40%
发文量
226
审稿时长
1.7 months
期刊介绍:
Nature Chemistry is a monthly journal that publishes groundbreaking and significant research in all areas of chemistry. It covers traditional subjects such as analytical, inorganic, organic, and physical chemistry, as well as a wide range of other topics including catalysis, computational and theoretical chemistry, and environmental chemistry.
The journal also features interdisciplinary research at the interface of chemistry with biology, materials science, nanotechnology, and physics. Manuscripts detailing such multidisciplinary work are encouraged, as long as the central theme pertains to chemistry.
Aside from primary research, Nature Chemistry publishes review articles, news and views, research highlights from other journals, commentaries, book reviews, correspondence, and analysis of the broader chemical landscape. It also addresses crucial issues related to education, funding, policy, intellectual property, and the societal impact of chemistry.
Nature Chemistry is dedicated to ensuring the highest standards of original research through a fair and rigorous review process. It offers authors maximum visibility for their papers, access to a broad readership, exceptional copy editing and production standards, rapid publication, and independence from academic societies and other vested interests.
Overall, Nature Chemistry aims to be the authoritative voice of the global chemical community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


