Carbon Dioxide-Induced Separations of Terephthalic Acid from Aqueous Disodium Terephthalate Solutions for Polyester Upcycling
IF 7.3
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Depolymerization of polyethylene terephthalate (PET) with sodium hydroxide has been shown to be highly productive and selective toward disodium terephthalate (Na2TPA). However, the traditional method to recover terephthalic acid (TPA) from aqueous solution uses strong inorganic acids. As a potential sustainable alternative, gaseous carbon dioxide (CO2) may be used as an acid switch for the recovery of pure TPA and the capture of CO2. TPA yields up to 94% were verified experimentally. Low temperatures and higher pressures, i.e., high CO2 solubility, favor the recovery of terephthalic acid. A preliminary sustainability assessment indicates that the choice of CO2 over HCl may lead to fewer environmental impacts.
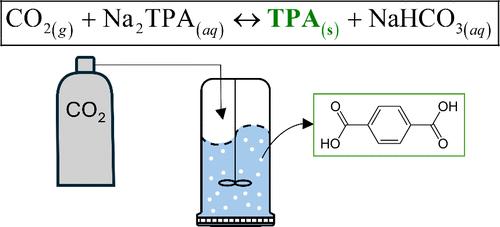
二氧化碳诱导聚酯升级回收中对苯二甲酸与对苯二甲酸二钠溶液的分离
聚对苯二甲酸乙二醇酯(PET)与氢氧化钠解聚已被证明是高产和选择性对苯二甲酸二钠(Na2TPA)。然而,传统的从水溶液中回收对苯二甲酸(TPA)的方法是使用强无机酸。作为一种潜在的可持续替代品,气态二氧化碳(CO2)可以用作回收纯TPA和捕获CO2的酸开关。实验验证了TPA产率可达94%。低温和高压,即CO2溶解度高,有利于对苯二甲酸的回收。一项初步的可持续性评估表明,选择CO2而不是HCl可能会减少对环境的影响。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Sustainable Chemistry & Engineering
CHEMISTRY, MULTIDISCIPLINARY-ENGINEERING, CHEMICAL
CiteScore
13.80
自引率
4.80%
发文量
1470
审稿时长
1.7 months
期刊介绍:
ACS Sustainable Chemistry & Engineering is a prestigious weekly peer-reviewed scientific journal published by the American Chemical Society. Dedicated to advancing the principles of green chemistry and green engineering, it covers a wide array of research topics including green chemistry, green engineering, biomass, alternative energy, and life cycle assessment.
The journal welcomes submissions in various formats, including Letters, Articles, Features, and Perspectives (Reviews), that address the challenges of sustainability in the chemical enterprise and contribute to the advancement of sustainable practices. Join us in shaping the future of sustainable chemistry and engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


