Boosting Supramolecular Gelation Efficiency and Properties: Ionic Strength as a Key to Superior Hydrogels
IF 3.9
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
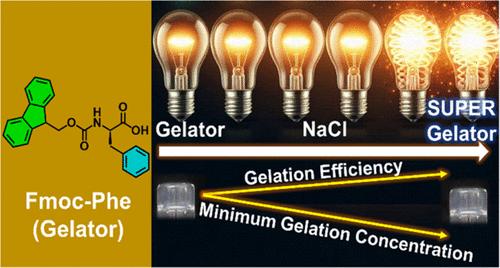
Controlling the minimum gelation concentration (MGC) of low molecular weight (LMW) hydrogelators is a key for modulating gel properties, such as mechanical strength, viscoelasticity, and stability, which are crucial for applications ranging from drug delivery to tissue engineering. However, tweaking the MGC under specific conditions, such as pH and/or temperature, poses a considerable challenge. Herein, we varied the ionic strength of buffer solutions using NaCl for several LMW hydrogelators, including Fmoc-Phe, Fmoc-Tyr, Fmoc-Trp, Fmoc-Met, and Fmoc-Cha, and assessed their gelation efficiency at pH 7.4 and ambient temperature. Interestingly, Fmoc-Phe demonstrated a ∼67% (3-fold) MGC reduction, from 0.24 to 0.08 wt %, at 500 mM NaCl, transforming it a “super hydrogelator” (MGC < 0.1 wt %), while Fmoc-Trp showed 60% MGC reduction. Higher ionic strength effectively shields the electrostatic repulsion between negatively charged (−COO–) groups on the Fmoc-Phe, promoting closer aggregation and more efficient self-assembly and allowing for gelation at lower concentrations. In contrast, Fmoc-Met, Fmoc-Cha, and Fmoc-Tyr precipitated in the presence of NaCl, suggesting that NaCl specifically modulates the MGC of Fmoc-amino acid gelators containing unsubstituted aromatic side chains. Furthermore, these results indicate that cation-π interactions likely play a role, alongside carboxylic acid neutralization. While Fmoc-Phe forms gels in the presence of other monovalent cations, it does not form a hydrogel in the presence of divalent (CaCl2) and trivalent (AlCl3), indicating that enhancement of hydrogelation is specific to monovalent cations. Although the fibrillar structure of Fmoc-Phe hydrogels remained consistent, addition of NaCl increased fibril stickiness, creating densely packed networks that modulate the mechanical strength. Unlike typical cases where increased ionic strength leads to precipitation, Fmoc-Phe gelation at high NaCl concentrations (150–500 mM) is significant, yielding a robust supramolecular hydrogel that remains stable in high ionic-strength environments. This outcome suggests that ionic strength could be a valuable factor to enhance the efficient gelation of LMW hydrogelators.
提高超分子凝胶效率和性能:离子强度是制备优质水凝胶的关键
控制低分子量(LMW)凝胶剂的最小凝胶浓度(MGC)是调节凝胶性能的关键,如机械强度、粘弹性和稳定性,这对于从药物输送到组织工程的应用至关重要。然而,在特定条件下(如pH和/或温度)调整MGC会带来相当大的挑战。在此,我们使用NaCl改变了几种LMW凝胶剂缓冲溶液的离子强度,包括Fmoc-Phe、Fmoc-Tyr、Fmoc-Trp、Fmoc-Met和Fmoc-Cha,并评估了它们在pH 7.4和环境温度下的凝胶效率。有趣的是,在500 mM NaCl下,Fmoc-Phe显示出约67%(3倍)的MGC减少,从0.24 wt %降至0.08 wt %,将其转化为“超级凝胶”(MGC <;0.1 wt %),而Fmoc-Trp显示60%的MGC减少。较高的离子强度有效地屏蔽了Fmoc-Phe上带负电荷(- COO -)基团之间的静电斥力,促进了更紧密的聚集和更有效的自组装,并允许在较低浓度下进行凝胶化。相比之下,Fmoc-Met、Fmoc-Cha和Fmoc-Tyr在NaCl存在下沉淀,表明NaCl特异性调节含有未取代芳侧链的fmoc -氨基酸凝胶的MGC。此外,这些结果表明阳离子-π相互作用可能与羧酸中和作用一起起作用。虽然Fmoc-Phe在其他一价阳离子存在下形成凝胶,但在二价(CaCl2)和三价(AlCl3)存在下不会形成水凝胶,这表明水凝胶化的增强是一价阳离子特异性的。虽然Fmoc-Phe水凝胶的纤维结构保持一致,但NaCl的加入增加了纤维的粘性,形成密集的排列网络,从而调节了机械强度。与离子强度增加导致沉淀的典型情况不同,在高NaCl浓度(150-500 mM)下,Fmoc-Phe凝胶作用显著,产生了强大的超分子水凝胶,在高离子强度环境下保持稳定。这一结果表明,离子强度可能是一个有价值的因素,以提高高效凝胶的LMW氢化剂。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Langmuir
化学-材料科学:综合
CiteScore
6.50
自引率
10.30%
发文量
1464
审稿时长
2.1 months
期刊介绍:
Langmuir is an interdisciplinary journal publishing articles in the following subject categories:
Colloids: surfactants and self-assembly, dispersions, emulsions, foams
Interfaces: adsorption, reactions, films, forces
Biological Interfaces: biocolloids, biomolecular and biomimetic materials
Materials: nano- and mesostructured materials, polymers, gels, liquid crystals
Electrochemistry: interfacial charge transfer, charge transport, electrocatalysis, electrokinetic phenomena, bioelectrochemistry
Devices and Applications: sensors, fluidics, patterning, catalysis, photonic crystals
However, when high-impact, original work is submitted that does not fit within the above categories, decisions to accept or decline such papers will be based on one criteria: What Would Irving Do?
Langmuir ranks #2 in citations out of 136 journals in the category of Physical Chemistry with 113,157 total citations. The journal received an Impact Factor of 4.384*.
This journal is also indexed in the categories of Materials Science (ranked #1) and Multidisciplinary Chemistry (ranked #5).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


