An engineered α1β1 integrin-mediated FcγRI signaling component to control enhanced CAR macrophage activation and phagocytosis
IF 10.5
1区 医学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
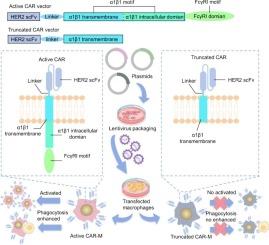
Treatment of solid tumors remains difficult, and therefore there has been increased focus on chimeric antigen receptor macrophages (CAR-M) to challenge solid tumors. However, CAR domain design of of adoptive cell therapy, which leads to differences in antitumor activity and triggered antitumor potential, remains poorly understood for macrophages. We developed an α1β1 integrin-mediated Fc-gamma receptor I (FcγRI) signaling component for CAR-M specific activation and its antitumor potential. We evaluated CAR-M effects with α1β1 integrin-mediated FcγRI signaling (ACT CAR-M) on the activation and antitumor phagocytic response of macrophages in vitro. Subcutaneous tumor model in BALB/c mice and carcinomatosis model in immunodeficient mice were used to test the antitumor effect of ACT CAR-M compared with CD3ζ CAR-M. The α1β1 integrin-mediated FcγRI signaling engagement of CAR-M was associated with enhanced macrophage activation and specific phagocytosis in primary human macrophages, and significantly improved tumor control and survival in multiple cancer models when compared to CD3ζ CAR-M. RNA-sequencing suggested that α1β1 integrin-mediated FcγRI engagement increased antitumor immunity by enhancing pro-inflammatory M1 phenotype-associated pathways, such as Toll-like receptor signaling, tumor necrosis factor signaling, and IL-17 signaling. α1β1 integrin-mediated FcγRI signaling engagement markedly enhanced antitumor effects of CAR-M immunotherapy, which is proposed as an advanced engineering CAR domain material to expand the clinical application of CAR-M.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Controlled Release
医学-化学综合
CiteScore
18.50
自引率
5.60%
发文量
700
审稿时长
39 days
期刊介绍:
The Journal of Controlled Release (JCR) proudly serves as the Official Journal of the Controlled Release Society and the Japan Society of Drug Delivery System.
Dedicated to the broad field of delivery science and technology, JCR publishes high-quality research articles covering drug delivery systems and all facets of formulations. This includes the physicochemical and biological properties of drugs, design and characterization of dosage forms, release mechanisms, in vivo testing, and formulation research and development across pharmaceutical, diagnostic, agricultural, environmental, cosmetic, and food industries.
Priority is given to manuscripts that contribute to the fundamental understanding of principles or demonstrate the advantages of novel technologies in terms of safety and efficacy over current clinical standards. JCR strives to be a leading platform for advancements in delivery science and technology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


