Membrane mechanics dictate axonal pearls-on-a-string morphology and function
IF 21.2
1区 医学
Q1 NEUROSCIENCES
引用次数: 0
Abstract
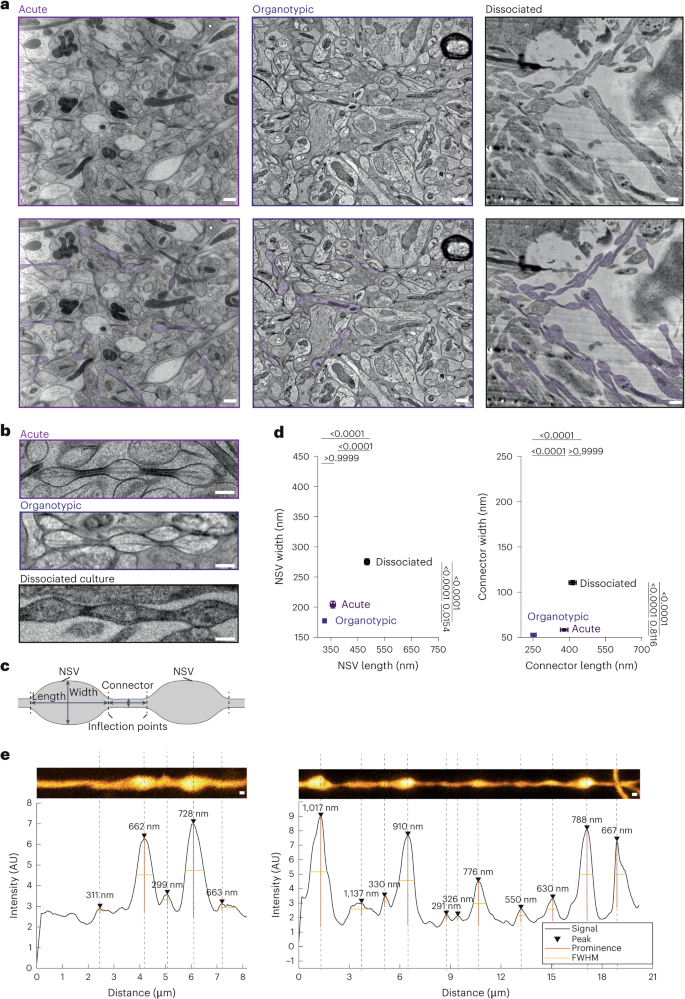
Axons are ultrathin membrane cables that are specialized for the conduction of action potentials. Although their diameter is variable along their length, how their morphology is determined is unclear. Here, we demonstrate that unmyelinated axons of the mouse central nervous system have nonsynaptic, nanoscopic varicosities ~200 nm in diameter repeatedly along their length interspersed with a thin cable ~60 nm in diameter like pearls-on-a-string. In silico modeling suggests that this axon nanopearling can be explained by membrane mechanical properties. Treatments disrupting membrane properties, such as hyper- or hypotonic solutions, cholesterol removal and nonmuscle myosin II inhibition, alter axon nanopearling, confirming the role of membrane mechanics in determining axon morphology. Furthermore, neuronal activity modulates plasma membrane cholesterol concentration, leading to changes in axon nanopearls and causing slowing of action potential conduction velocity. These data reveal that biophysical forces dictate axon morphology and function, and modulation of membrane mechanics likely underlies unmyelinated axonal plasticity. Axons have always been assumed to be cylindrical. Using in silico modeling and cryopreservation of tissues, Griswold et al. demonstrate that unmyelinated axons of the mammalian central nervous system exhibit pearls-on-a-string morphology through their entire length.

膜力学决定轴突串珠的形态和功能
轴突是一种超薄的薄膜电缆,专门用于传导动作电位。虽然它们的直径随长度变化,但它们的形态是如何确定的还不清楚。在这里,我们证明了小鼠中枢神经系统的无髓鞘轴突具有直径约200 nm的非突触性纳米级的静脉曲张,沿着它们的长度重复分布着直径约60 nm的细电缆,就像串在绳子上的珍珠。计算机模拟表明,这种轴突纳米珠状现象可以用膜的力学特性来解释。破坏膜特性的处理,如高或低渗溶液、胆固醇去除和非肌肉肌球蛋白II抑制,改变轴突纳米珠状,证实了膜力学在决定轴突形态中的作用。此外,神经元活动调节质膜胆固醇浓度,导致轴突纳米珠的变化,导致动作电位传导速度减慢。这些数据表明,生物物理力决定了轴突的形态和功能,而膜力学的调节可能是无髓鞘轴突可塑性的基础。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature neuroscience
医学-神经科学
CiteScore
38.60
自引率
1.20%
发文量
212
审稿时长
1 months
期刊介绍:
Nature Neuroscience, a multidisciplinary journal, publishes papers of the utmost quality and significance across all realms of neuroscience. The editors welcome contributions spanning molecular, cellular, systems, and cognitive neuroscience, along with psychophysics, computational modeling, and nervous system disorders. While no area is off-limits, studies offering fundamental insights into nervous system function receive priority.
The journal offers high visibility to both readers and authors, fostering interdisciplinary communication and accessibility to a broad audience. It maintains high standards of copy editing and production, rigorous peer review, rapid publication, and operates independently from academic societies and other vested interests.
In addition to primary research, Nature Neuroscience features news and views, reviews, editorials, commentaries, perspectives, book reviews, and correspondence, aiming to serve as the voice of the global neuroscience community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


