Selenium removal from water using modified biochar: A critical review and insights to adsorption mechanisms through computational analyses
IF 6.3
2区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
Selenium (Se) is an essential micronutrient for human and animal health and becomes toxic at elevated concentrations. Escalating Se concentration in water bodies has become a growing serious global issue. Biochar (BC) is recognized as a green adsorbent for the removal of potentially toxic metals including Se. However, the practical application of pristine BC is hindered by its limited efficacy for Se oxyanions. To improve the removal efficiency of BC for Se oxyanions various techniques have been employed to modify BC and enrich it with a range of physiochemical attributes. This systematic review aimed to critically analyze the efficacy of various modified biochar (MBC) for Se oxyanions removal from water, comprehend their adsorption mechanisms at the molecular level, and evaluate adsorption influencing parameters. The literature (2010–2024) showed that Se oxyanions removal using MBCs better fitted with Langmuir isotherm model and their removal kinetics followed the pseudo-second-order model. Moreover, the solution pH plays a critical role in the removal of Se oxyanions using MBCs; maximum removal was reported under acidic conditions. The potential removal mechanisms include surface complexation, reduction, and electrostatic interactions. Simulations (molecular dynamics and density functional theory) were conducted to elucidate the removal mechanism at the molecular scale and demonstrate alignment between experimental and computational findings. Moreover, the Bader charge analysis was employed, and its findings revealed the transfer of electrons from the surface of MBCs to Se oxyanions. This review delivers a simulation methodology for screening MBCs for removal of Se oxyanions from water prior to experimental efforts.
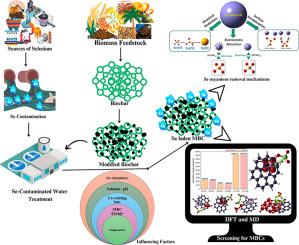
利用改性生物炭从水中去除硒:通过计算分析对吸附机制的重要回顾和见解
硒(Se)是人类和动物健康必需的微量营养素,浓度升高会产生毒性。水体中硒浓度不断升高已成为日益严重的全球性问题。生物炭(BC)被认为是一种绿色吸附剂,可以去除包括硒在内的潜在有毒金属。然而,原始BC的实际应用受到其对硒氧离子的有限功效的阻碍。为了提高BC对硒氧离子的去除效率,人们采用了各种技术对BC进行改性,使其具有一系列的理化性质。本系统综述旨在批判性地分析各种改性生物炭(MBC)去除水中硒氧离子的效果,了解其在分子水平上的吸附机理,并评估吸附的影响参数。文献(2010-2024)表明,MBCs对Se氧阴离子的去除更符合Langmuir等温线模型,其去除动力学符合拟二阶模型。此外,溶液pH对MBCs去除Se氧阴离子起关键作用;在酸性条件下脱除效果最大。潜在的去除机制包括表面络合、还原和静电相互作用。通过模拟(分子动力学和密度泛函理论)来阐明分子尺度上的去除机制,并证明实验和计算结果之间的一致性。此外,采用Bader电荷分析,其结果揭示了电子从MBCs表面转移到Se氧离子。本综述提供了一种模拟方法,用于在实验之前筛选MBCs以去除水中的硒氧阴离子。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of water process engineering
Biochemistry, Genetics and Molecular Biology-Biotechnology
CiteScore
10.70
自引率
8.60%
发文量
846
审稿时长
24 days
期刊介绍:
The Journal of Water Process Engineering aims to publish refereed, high-quality research papers with significant novelty and impact in all areas of the engineering of water and wastewater processing . Papers on advanced and novel treatment processes and technologies are particularly welcome. The Journal considers papers in areas such as nanotechnology and biotechnology applications in water, novel oxidation and separation processes, membrane processes (except those for desalination) , catalytic processes for the removal of water contaminants, sustainable processes, water reuse and recycling, water use and wastewater minimization, integrated/hybrid technology, process modeling of water treatment and novel treatment processes. Submissions on the subject of adsorbents, including standard measurements of adsorption kinetics and equilibrium will only be considered if there is a genuine case for novelty and contribution, for example highly novel, sustainable adsorbents and their use: papers on activated carbon-type materials derived from natural matter, or surfactant-modified clays and related minerals, would not fulfil this criterion. The Journal particularly welcomes contributions involving environmentally, economically and socially sustainable technology for water treatment, including those which are energy-efficient, with minimal or no chemical consumption, and capable of water recycling and reuse that minimizes the direct disposal of wastewater to the aquatic environment. Papers that describe novel ideas for solving issues related to water quality and availability are also welcome, as are those that show the transfer of techniques from other disciplines. The Journal will consider papers dealing with processes for various water matrices including drinking water (except desalination), domestic, urban and industrial wastewaters, in addition to their residues. It is expected that the journal will be of particular relevance to chemical and process engineers working in the field. The Journal welcomes Full Text papers, Short Communications, State-of-the-Art Reviews and Letters to Editors and Case Studies
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


