“Tennis racket” hydrogel electrolytes to synchronously regulate cathode and anode of zinc-iodine batteries
IF 13.1
1区 化学
Q1 Energy
引用次数: 0
Abstract
Aqueous zinc-iodine (Zn-I2) batteries show great potential as energy storage candidates due to their high-safety and low-cost, but confronts hydrogen evolution reaction (HER) and dendrite growth at anode side and polyiodide shuttling at cathode side. Herein, “tennis racket” (TR) hydrogel electrolytes were prepared by the co-polymerization and co-blending of polyacrylamide (PAM), sodium lignosulfonate (SL), and sodium alginate (SA) to synchronously regulate cathode and anode of Zn-I2 batteries. “Gridline structure” of TR can induce the uniform transportation of Zn2+ ions through the coordination effect to hinder HER and dendrite growth at anode side, as well as hit I3− ions as “tennis” via the strong repulsion force to avoid shuttle effect at cathode side. The synergistic effect of TR electrolyte endows Zn-Zn symmetric battery with high cycling stability over 4500 h and Zn-I2 cell with the stably cycling life of 15000 cycles at 5 A g−1, outperforming the reported works. The practicability of TR electrolyte is verified by flexible Zn-I2 pouch battery. This work opens a route to synchronously regulate cathode and anode to enhance the electrochemical performance of Zn-I2 batteries.
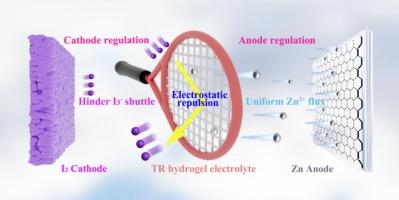
“网球拍”水凝胶电解质同步调节锌碘电池正极和阳极
水相锌碘(Zn-I2)电池以其高安全性和低成本的优点显示出巨大的储能潜力,但在阳极侧存在析氢反应(HER)和枝晶生长问题,在阴极侧存在多碘化物穿梭问题。采用聚丙烯酰胺(PAM)、木质素磺酸钠(SL)和海藻酸钠(SA)共聚共混制备了“网球拍”(TR)水凝胶电解质,用于同步调节Zn-I2电池的正极和阳极。TR的“网格线结构”可以通过配位效应诱导Zn2+离子均匀输运,阻碍阳极侧HER和枝晶的生长,并通过强大的斥力将I3−离子打成“网球”,避免阴极侧的穿梭效应。TR电解液的协同作用使Zn-Zn对称电池具有超过4500 h的高循环稳定性,Zn-I2电池在5 A g−1下具有15000次的稳定循环寿命,优于报道的工作。用柔性锌- i2袋电池验证了TR电解液的实用性。本工作开辟了同步调节阴极和阳极以提高锌- i2电池电化学性能的途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Energy Chemistry
CHEMISTRY, APPLIED-CHEMISTRY, PHYSICAL
CiteScore
19.10
自引率
8.40%
发文量
3631
审稿时长
15 days
期刊介绍:
The Journal of Energy Chemistry, the official publication of Science Press and the Dalian Institute of Chemical Physics, Chinese Academy of Sciences, serves as a platform for reporting creative research and innovative applications in energy chemistry. It mainly reports on creative researches and innovative applications of chemical conversions of fossil energy, carbon dioxide, electrochemical energy and hydrogen energy, as well as the conversions of biomass and solar energy related with chemical issues to promote academic exchanges in the field of energy chemistry and to accelerate the exploration, research and development of energy science and technologies.
This journal focuses on original research papers covering various topics within energy chemistry worldwide, including:
Optimized utilization of fossil energy
Hydrogen energy
Conversion and storage of electrochemical energy
Capture, storage, and chemical conversion of carbon dioxide
Materials and nanotechnologies for energy conversion and storage
Chemistry in biomass conversion
Chemistry in the utilization of solar energy
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


