The electrochemical performance deterioration mechanism of LiNi0.83Mn0.05Co0.12O2 in aqueous slurry and a mitigation strategy
IF 13.1
1区 化学
Q1 Energy
引用次数: 0
Abstract
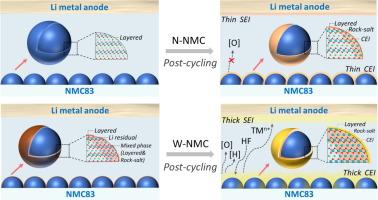
Integrating high-nickel layered oxide cathodes with aqueous slurry electrode preparation routes holds the potential to simultaneously meet the demands for high energy density and low-cost production of lithium-ion batteries. However, the influence of dual exposure to air and liquid water as well as the heating treatment during aqueous slurry electrode processing on the high-nickel layered oxide electrode is yet to be understood. In this study, we systematically investigate the structural evolution and electrochemical behaviors when LiNi0.83Mn0.05Co0.12O2 (NMC83) is subjected to aqueous slurry processing. It was observed that the crystal structure near the surface of NMC83 is partially reconstructed to contain a mixture of rock-salt and layered phases when exposed to water, leading to the deteriorated rate capability of the NMC83 electrodes. This partial surface reconstruction layer completely converts into a pure rock-salt phase upon cycling, accompanied by the release of O2, Ni leaching, catalyzed decomposition of the electrolyte, and the formation of a thick cathode electrolyte interphase layer. The byproducts of the electrolyte and dissolved Ni could shuttle to the Li metal side, causing a crosstalk effect that results in a thick and unstable solid electrolyte interphase layer on the Li surface. These in combination severely undermined the cycling stability of the NMC83 electrodes obtained from the aqueous slurry. A mitigation strategy using molecular self-assembly technique was demonstrated to enhance the surface stability of water-treated NMC83. Our findings offer new insights for tailoring ambient environment stability and aqueous slurry processability for ultra-high nickel layered oxide and other water-sensitive cathode materials.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Energy Chemistry
CHEMISTRY, APPLIED-CHEMISTRY, PHYSICAL
CiteScore
19.10
自引率
8.40%
发文量
3631
审稿时长
15 days
期刊介绍:
The Journal of Energy Chemistry, the official publication of Science Press and the Dalian Institute of Chemical Physics, Chinese Academy of Sciences, serves as a platform for reporting creative research and innovative applications in energy chemistry. It mainly reports on creative researches and innovative applications of chemical conversions of fossil energy, carbon dioxide, electrochemical energy and hydrogen energy, as well as the conversions of biomass and solar energy related with chemical issues to promote academic exchanges in the field of energy chemistry and to accelerate the exploration, research and development of energy science and technologies.
This journal focuses on original research papers covering various topics within energy chemistry worldwide, including:
Optimized utilization of fossil energy
Hydrogen energy
Conversion and storage of electrochemical energy
Capture, storage, and chemical conversion of carbon dioxide
Materials and nanotechnologies for energy conversion and storage
Chemistry in biomass conversion
Chemistry in the utilization of solar energy
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


