High-yield pentanes-plus production via hydrogenation of carbon dioxide: Revealing new roles of zirconia as promoter of iron catalyst with long-term stability
IF 13.1
1区 化学
Q1 Energy
引用次数: 0
Abstract
The metal oxide promoter decisively influences the overall performance of Fe catalysts in the direct hydrogenation of CO2 to C5+ hydrocarbons. However, the roles of metal oxide promoter for Fe catalysts, particularly ZrO2, have rarely been investigated. To plug this knowledge gap, a new Fe catalyst promoted with Na and partially reduced ZrOx (Na-FeZrOx-9) was developed in this study; the catalyst helped produce C5+ hydrocarbons in remarkably high yield (26.3% at 360 °C). In contrast to ZrOx-free Fe-oxide, Na-FeZrOx-9 exhibited long-term stability for CO2 hydrogenation (750 h on-stream). The findings revealed multiple roles of ZrOx. Notably, ZrOx decorated the Fe-oxide particles after calcination, thereby suppressing excess particle aggregation during the reaction, and acted as a “coke remover” to eliminate the carbon deposited on the catalyst surface. Additionally, oxygen vacancy (Ov) sites in ZrOx and electron transfer from ZrOx to Fe sites facilitated the adsorption of CO2 at the Zr-Fe interface.
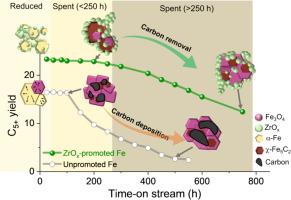
二氧化碳加氢高产正戊烷:揭示氧化锆促进铁催化剂长期稳定的新作用
在CO2直接加氢制C5+烃过程中,金属氧化物助剂对Fe催化剂的整体性能有决定性影响。然而,金属氧化物促进剂的作用,特别是ZrO2,很少被研究。为了填补这一空白,本研究开发了一种新的以Na和部分还原的ZrOx促进的Fe催化剂(Na- fezrox -9);在360°C时,C5+碳氢化合物的产率高达26.3%。与不含zrox的Fe-oxide相比,Na-FeZrOx-9在CO2加氢过程中表现出长期稳定性(750 h)。研究结果揭示了ZrOx的多重作用。值得注意的是,ZrOx在煅烧后修饰了氧化铁颗粒,从而抑制了反应过程中过量的颗粒聚集,并起到了“除焦剂”的作用,消除了沉积在催化剂表面的碳。此外,ZrOx中的氧空位(Ov)位点和ZrOx向Fe位点的电子转移促进了CO2在Zr-Fe界面的吸附。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Energy Chemistry
CHEMISTRY, APPLIED-CHEMISTRY, PHYSICAL
CiteScore
19.10
自引率
8.40%
发文量
3631
审稿时长
15 days
期刊介绍:
The Journal of Energy Chemistry, the official publication of Science Press and the Dalian Institute of Chemical Physics, Chinese Academy of Sciences, serves as a platform for reporting creative research and innovative applications in energy chemistry. It mainly reports on creative researches and innovative applications of chemical conversions of fossil energy, carbon dioxide, electrochemical energy and hydrogen energy, as well as the conversions of biomass and solar energy related with chemical issues to promote academic exchanges in the field of energy chemistry and to accelerate the exploration, research and development of energy science and technologies.
This journal focuses on original research papers covering various topics within energy chemistry worldwide, including:
Optimized utilization of fossil energy
Hydrogen energy
Conversion and storage of electrochemical energy
Capture, storage, and chemical conversion of carbon dioxide
Materials and nanotechnologies for energy conversion and storage
Chemistry in biomass conversion
Chemistry in the utilization of solar energy
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


