Engineering Nicotiana benthamiana for production of active cannabinoid synthases via secretory pathway optimization
Q1 Immunology and Microbiology
引用次数: 0
Abstract
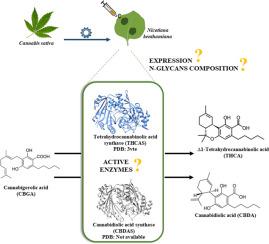
The production of cannabinoid compounds such as Δ9-tetrahydrocannabinol (THC), cannabidiol (CBD) and cannabichromene (CBC) with potential pharmaceutical applications is growing sharply. However, challenges such as the low yield of minor cannabinoids, legal restrictions on cultivation, and the complexity and cost of purification from the Cannabis sativa plant necessitate a biotechnological approach. Since the biosynthetic pathway is disclosed, cannabinoids have been produced in yeast, insect cells and plants mainly by the heterologous expression of tetrahydrocannabinol acid synthase (THCAS). THCAS and cannabidiolic acid synthase (CBDAS) use cannabigerolic acid (CBGA) as a substrate. In this study, we transiently expressed recombinant forms of THCAS and CBDAS in leaves of Nicotiana benthamiana. Our results demonstrate that efficient expression in the secretory pathway relies on replacing the endogenous signal peptide with a heterologous one. Both proteins were successfully secreted to the apoplast. MS-based analysis of the purified proteins revealed that they are heavily glycosylated with mainly Golgi-processed complex type N-glycans. In planta enzymatic removal of N-glycans indicated that glycosylation plays a role for CBDAS protein folding or stability. Finally, in vitro assays with CBGA showed that the plant-made recombinant CBDAS and THCAS are enzymatically active.
通过分泌途径优化,工程烟叶生产活性大麻素合成酶
具有潜在制药应用价值的大麻素化合物如Δ9-tetrahydrocannabinol (THC)、大麻二酚(CBD)和大麻红素(CBC)的生产正在急剧增长。然而,诸如少量大麻素产量低、对种植的法律限制以及从大麻植物中提纯的复杂性和成本等挑战需要采用生物技术方法。自该生物合成途径被披露以来,大麻素主要通过四氢大麻酚酸合成酶(THCAS)的异源表达在酵母、昆虫细胞和植物中产生。THCAS和大麻二酚酸合成酶(CBDAS)使用大麻二酚酸(CBGA)作为底物。在本研究中,我们在烟叶中瞬时表达了THCAS和CBDAS的重组形式。我们的研究结果表明,分泌途径的有效表达依赖于用外源信号肽替代内源信号肽。两种蛋白都成功地分泌到外质体中。纯化蛋白的质谱分析显示,它们主要被高尔基加工的n -聚糖严重糖基化。在植物中n -聚糖的酶解表明糖基化对CBDAS蛋白的折叠或稳定性起作用。最后,体外CBGA实验表明,植物合成的重组CBDAS和THCAS具有酶活性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Biotechnology Reports
Immunology and Microbiology-Applied Microbiology and Biotechnology
CiteScore
15.80
自引率
0.00%
发文量
79
审稿时长
55 days
期刊介绍:
Biotechnology Reports covers all aspects of Biotechnology particularly those reports that are useful and informative and that will be of value to other researchers in related fields. Biotechnology Reports loves ground breaking science, but will also accept good science that can be of use to the biotechnology community. The journal maintains a high quality peer review where submissions are considered on the basis of scientific validity and technical quality. Acceptable paper types are research articles (short or full communications), methods, mini-reviews, and commentaries in the following areas: Healthcare and pharmaceutical biotechnology Agricultural and food biotechnology Environmental biotechnology Molecular biology, cell and tissue engineering and synthetic biology Industrial biotechnology, biofuels and bioenergy Nanobiotechnology Bioinformatics & systems biology New processes and products in biotechnology, bioprocess engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


