Competitive adsorption of two phenolic pollutants compounds using a novel biosorbent: Analytics (HPLC), Statistical (experimental design), and theoretical studies (DFT)
IF 4.9
2区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
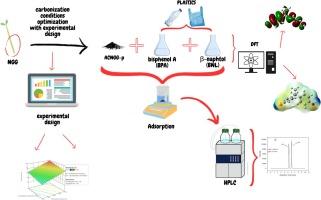
In this study, we focused on a novel agricultural waste material, specifically a plant known as Nicotiana glauca Graham (NgG), which is highly abundant in Morocco. The investigation involved testing four activating agents H3PO4, H2SO4, NaOH, and ZnCl2 on Nicotiana glauca Graham (NgG) to assess their effects. By employing an experimental design, we successfully determined the optimal conditions for this activation process. The resulting activated carbon was then evaluated for its effectiveness in removing two phenolic pollutants, Bisphenol A (BPA) and β-naphthol (BNL). Analysis using FTIR revealed various functional groups on the activated carbon surface, including P = O, P-O-C aromatics, and O-H groups, which played a crucial role in the adsorption of BPA and BNL. XRD analysis indicated that the optimal adsorbent was amorphous, while Zeta potential measurements showed a significant decrease in pollutant removal rates after reaching a pH level of nearly 10. The activated carbon produced from H3PO4 exhibited a surface area of 1078 m2/g. Experimental adsorption results at the highest removal rate showed qBPAs = 125.82 mg/g for BPA and qBNLs = 62.82 mg/g for BNL in individual mode, while in the mixed mode, qBPAm = 16.22 mg/g for BPA and qBNLm = 16.04 mg/g for BNL were observed. To enhance our study, we utilized Density Functional Theory (DFT) calculations to identify the most electrophilic and nucleophilic regions on BPA and BNL. The analysis highlighted the hydroxyl groups (–OH) of BNL and BPA as the most significant negative zones, crucial for understanding the underlying mechanisms and explaining the experimental observations. In the isotherm analysis, we identified the Temkin model in a singular mode and the Langmuir model in a mixture mode, showcasing a notable distinction between the two modes.
新型生物吸附剂对两种酚类污染物化合物的竞争吸附:分析(HPLC)、统计(实验设计)和理论研究(DFT)
在这项研究中,我们专注于一种新的农业废弃物,特别是一种被称为Nicotiana glauca Graham (NgG)的植物,它在摩洛哥非常丰富。本研究采用四种活化剂H3PO4、H2SO4、NaOH和ZnCl2对烟叶(Nicotiana glauca Graham, NgG)进行试验,评价其对烟叶(Nicotiana glauca Graham, NgG)的作用。通过实验设计,我们成功地确定了该活化过程的最佳条件。然后对所得活性炭去除双酚A (BPA)和β-萘酚(BNL)两种酚类污染物的效果进行了评价。FTIR分析表明,活性炭表面存在P = O、P-O- c芳烃和O- h等多种官能团,它们对BPA和BNL的吸附起着至关重要的作用。XRD分析表明,最佳吸附剂为无定形,而Zeta电位测量表明,当pH值接近10时,污染物去除率显著下降。由H3PO4制备的活性炭的表面积为1078 m2/g。实验结果表明,在单独模式下,双酚a的qbpa = 125.82 mg/g, BNL的qBNLs = 62.82 mg/g,而在混合模式下,双酚a的qBPAm = 16.22 mg/g, BNL的qBNLm = 16.04 mg/g。为了加强我们的研究,我们利用密度泛函理论(DFT)计算确定了BPA和BNL上最亲电和亲核的区域。分析强调了BNL和BPA的羟基(-OH)是最显著的负区,这对于理解潜在机制和解释实验观察结果至关重要。在等温线分析中,我们确定了Temkin模型为单一模态,Langmuir模型为混合模态,显示了两种模态之间的显著区别。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Microchemical Journal
化学-分析化学
CiteScore
8.70
自引率
8.30%
发文量
1131
审稿时长
1.9 months
期刊介绍:
The Microchemical Journal is a peer reviewed journal devoted to all aspects and phases of analytical chemistry and chemical analysis. The Microchemical Journal publishes articles which are at the forefront of modern analytical chemistry and cover innovations in the techniques to the finest possible limits. This includes fundamental aspects, instrumentation, new developments, innovative and novel methods and applications including environmental and clinical field.
Traditional classical analytical methods such as spectrophotometry and titrimetry as well as established instrumentation methods such as flame and graphite furnace atomic absorption spectrometry, gas chromatography, and modified glassy or carbon electrode electrochemical methods will be considered, provided they show significant improvements and novelty compared to the established methods.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


