Efficacy of GPE− strain live attenuated vaccine and CP7_E2alf strain recombinant live vaccine (marker vaccine) against Japanese epidemic classical swine fever virus isolated in 2019 and DIVA discrimination ability of the marker vaccine
IF 2.2
3区 农林科学
Q1 VETERINARY SCIENCES
引用次数: 0
Abstract
Classical swine fever (CSF) re-emerged in Japan in 2018, with the epidemic virus identified as genotype 2.1, which is moderately virulent and more difficult to detect and control than the highly virulent strain. Domestic pigs were administered with GPE− strain live attenuated vaccine (GPE− vaccine) for outbreak management. CP7_E2alf strain recombinant live vaccine (marker vaccine), approved for differentiation of infected from vaccinated animals (DIVA), was considered optional for obtaining CSF-free country certification issued by the World Organization for Animal Health. This study aimed to assess the efficacy of both vaccines in pigs through experimental challenge tests and evaluate the DIVA ability of the marker vaccine using two enzyme-linked immunosorbent assay (ELISA) antibody detection kits. Results showed that both GPE− and marker vaccines were effective against the Japanese epidemic strain; however, the ability of the ELISA antibody detection kits to discriminate the marker vaccine was limited. Therefore, to achieve CSF-free certification using vaccines with DIVA functionality, alternative detection methods and enhancement of the sensitivity and specificity of ELISA kits are needed.
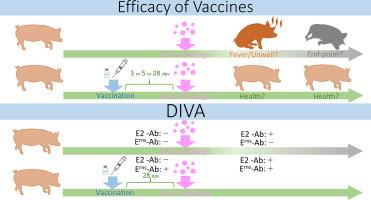
GPE−株减毒活疫苗和CP7_E2alf株重组活疫苗(标记疫苗)对2019年日本流行猪瘟病毒的免疫效果及标记疫苗的DIVA识别能力
经典猪瘟(CSF)于2018年在日本重新出现,该流行病毒被确定为基因型2.1,其毒性中等,比高毒性菌株更难检测和控制。用GPE -株减毒活疫苗(GPE -疫苗)对家猪进行疫情管理。CP7_E2alf株重组活疫苗(标记疫苗)被批准用于区分受感染动物和已接种动物(DIVA),被认为是获得世界动物卫生组织颁发的无csf国家认证的可选疫苗。本研究旨在通过实验性攻毒试验评估两种疫苗在猪体内的有效性,并使用两种酶联免疫吸附试验(ELISA)抗体检测试剂盒评估标记疫苗的DIVA能力。结果表明,GPE -和标记疫苗对日本流行毒株均有效;然而,ELISA抗体检测试剂盒区分标记疫苗的能力有限。因此,为了使用具有DIVA功能的疫苗获得无csf认证,需要替代检测方法并提高ELISA试剂盒的敏感性和特异性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Research in veterinary science
农林科学-兽医学
CiteScore
4.40
自引率
4.20%
发文量
312
审稿时长
75 days
期刊介绍:
Research in Veterinary Science is an International multi-disciplinary journal publishing original articles, reviews and short communications of a high scientific and ethical standard in all aspects of veterinary and biomedical research.
The primary aim of the journal is to inform veterinary and biomedical scientists of significant advances in veterinary and related research through prompt publication and dissemination. Secondly, the journal aims to provide a general multi-disciplinary forum for discussion and debate of news and issues concerning veterinary science. Thirdly, to promote the dissemination of knowledge to a broader range of professions, globally.
High quality papers on all species of animals are considered, particularly those considered to be of high scientific importance and originality, and with interdisciplinary interest. The journal encourages papers providing results that have clear implications for understanding disease pathogenesis and for the development of control measures or treatments, as well as those dealing with a comparative biomedical approach, which represents a substantial improvement to animal and human health.
Studies without a robust scientific hypothesis or that are preliminary, or of weak originality, as well as negative results, are not appropriate for the journal. Furthermore, observational approaches, case studies or field reports lacking an advancement in general knowledge do not fall within the scope of the journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


