Interfacial migration–diffusion and oil displacement mechanism of middle-phase microemulsion
IF 6.7
1区 工程技术
Q2 ENERGY & FUELS
引用次数: 0
Abstract
Research on the phase transition mechanism of surfactant-emulsified crude oil and the oil displacement mechanism of microemulsions remains relatively scarce. In this study, phase behavior experiments were conducted using three surfactant complex systems with crude oil. Gas chromatography was employed to quantitatively characterize the changes in the carbon components of the solution during the phase transition. The oil displacement mechanism of the microemulsions was determined based on oil displacement visualization images within a microfluidic chip. The results showed that the optimal complex electrolyte concentrations for forming middle-phase microemulsions were 1.70% for the fatty alcohol polyoxypropylene ether sulfate (AES) and long-chain alkylbenzene sulfonate (ABS) complex system, 1.40% for the emulsifier/petroleum sulfonate complex system, and 1.90% for interfacially obstructive surfactants. When oil–water ratios of 3:7, 4:6, 5:5, and 6:4 were tested, the optimal complex electrolyte concentrations for forming middle-phase microemulsions decreased with increasing oil content, narrowing the range of optimal concentrations. The solubilization range of the AES/ABS complex system was C9–C15, with a solubilization ratio of 22.67%. For the emulsifier/petroleum sulfonate complex system, the solubilization range was C10–C17, with a solubilization ratio of 6.27%. The solubilization range of the interfacially obstructive surfactants was C10–C18, with a solubilization ratio of 11.02%. Based on the dynamic oil displacement images collected within the microfluidic chip, the oil displacement mechanism of the microemulsions was determined as emulsification into small droplets and stripping, trapping, and activation of oil droplets that aggregate and migrate as oil bands. This study innovatively quantified the internal carbon components of middle-phase microemulsions, clarified the target carbon component ranges for solubilization by different surfactants, and revealed the migration–diffusion theory of phase transition in middle-phase microemulsions at the microscopic scale, filling a gap in theoretical research at the microscale. The findings of this study provide technical guidance and theoretical support for the large-scale field applications of surfactant complex systems.
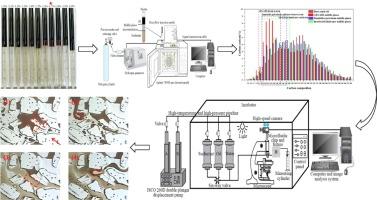
中相微乳液界面迁移扩散及驱油机理研究
对表面活性剂乳化原油的相变机理和微乳驱油机理的研究相对较少。本研究采用三种表面活性剂配合体系与原油进行了相行为实验。采用气相色谱法定量表征了溶液中碳组分在相变过程中的变化。基于微流控芯片内的驱油可视化图像,确定了微乳液的驱油机理。结果表明,形成中相微乳的最佳络合电解质浓度为:脂肪醇聚氧丙烯醚硫酸酯(AES)与长链烷基苯磺酸盐(ABS)络合体系为1.70%,乳化剂与石油磺酸盐络合体系为1.40%,界面阻碍表面活性剂为1.90%。当油水比为3:7、4:6、5:5和6:4时,形成中间相微乳的最佳复合电解质浓度随含油量的增加而降低,最佳浓度范围缩小。AES/ABS复合体系的增溶范围为c9 ~ c15,增溶率为22.67%。乳化剂/石油磺酸盐复合体系的增溶范围为c10 ~ c17,增溶比为6.27%。界面阻碍表面活性剂的增溶范围为c10 ~ c18,增溶率为11.02%。基于微流控芯片内采集的动态驱油图像,确定微乳液的驱油机理为乳化成小液滴,并对油滴进行剥离、捕集、活化,形成油带聚集迁移。本研究创新性地量化了中相微乳的内部碳组分,明确了不同表面活性剂增溶的目标碳组分范围,揭示了微观尺度上中相微乳相变的迁移-扩散理论,填补了微观尺度上理论研究的空白。研究结果为表面活性剂复合体系的大规模现场应用提供了技术指导和理论支持。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Fuel
工程技术-工程:化工
CiteScore
12.80
自引率
20.30%
发文量
3506
审稿时长
64 days
期刊介绍:
The exploration of energy sources remains a critical matter of study. For the past nine decades, fuel has consistently held the forefront in primary research efforts within the field of energy science. This area of investigation encompasses a wide range of subjects, with a particular emphasis on emerging concerns like environmental factors and pollution.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


