Potassium storage behavior and low-temperature performance of typical carbon anodes in potassium-ion hybrid capacitors enabled by Co-intercalation graphite chemistry
IF 10.5
2区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Carbon materials are widely explored as anodes for potassium-ion storage, yet the slow K+ desolvation process in electrolyte at low temperatures presents a kinetic limitation that impedes reliable operation in specific conditions. In this work, we systematically investigate the potassium storage behavior of four typical carbon materials—graphite, hard carbon, activated carbon, and graphene—in a 1 M KFSI-Diglyme electrolyte, highlighting a co-intercalation approach that significantly reduces the desolvation energy barrier. The formation of ternary graphite intercalation compounds (t-GICs) through co-intercalation in graphite introduces weak interlayer interactions between the graphite layers and solvated K+, which accelerate K+ diffusion in the anode, thereby enhancing reaction kinetics. This unique mechanism enables the graphite anode to deliver remarkable rate performance (98 mAh g−1 at 0.05 A g−1 and 76 mAh g−1 at 1 A g−1) even at −20 °C. Furthermore, potassium-ion hybrid capacitors (PICs) using the graphite anode achieve impressive cycling stability, with 88 % capacity retention after 2000 cycles at 2 A g−1 and a high power density of 11.1 kW kg−1 (57 Wh kg−1) at −20 °C. These findings provide key insights into the design of robust potassium-ion storage devices capable of sustaining high performance in low-temperature environments.
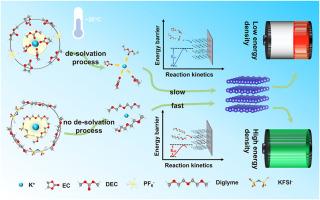
石墨化学共插层钾离子杂化电容器中典型碳阳极的储钾行为和低温性能
碳材料作为钾离子储存的阳极被广泛探索,但在低温下电解质中缓慢的K+脱溶过程存在动力学限制,阻碍了在特定条件下的可靠运行。在这项工作中,我们系统地研究了四种典型的碳材料——石墨、硬碳、活性炭和石墨烯——在1 M KFSI-Diglyme电解质中的钾储存行为,强调了一种显著降低脱溶能垒的共插层方法。通过石墨共插层形成三元石墨插层化合物(t-GICs),石墨层与溶剂化K+之间产生弱的层间相互作用,加速K+在阳极中的扩散,从而提高反应动力学。这种独特的机制使石墨阳极即使在- 20°C下也能提供显着的倍率性能(0.05 A g−1时98 mAh g−1,1 A g−1时76 mAh g−1)。此外,使用石墨阳极的钾离子混合电容器(PICs)实现了令人印象深刻的循环稳定性,在2 A g - 1下循环2000次后,其容量保持率为88%,在- 20°C下的高功率密度为11.1 kW kg - 1 (57 Wh kg - 1)。这些发现为设计能够在低温环境中保持高性能的坚固的钾离子存储装置提供了关键见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Carbon
工程技术-材料科学:综合
CiteScore
20.80
自引率
7.30%
发文量
0
审稿时长
23 days
期刊介绍:
The journal Carbon is an international multidisciplinary forum for communicating scientific advances in the field of carbon materials. It reports new findings related to the formation, structure, properties, behaviors, and technological applications of carbons. Carbons are a broad class of ordered or disordered solid phases composed primarily of elemental carbon, including but not limited to carbon black, carbon fibers and filaments, carbon nanotubes, diamond and diamond-like carbon, fullerenes, glassy carbon, graphite, graphene, graphene-oxide, porous carbons, pyrolytic carbon, and other sp2 and non-sp2 hybridized carbon systems. Carbon is the companion title to the open access journal Carbon Trends. Relevant application areas for carbon materials include biology and medicine, catalysis, electronic, optoelectronic, spintronic, high-frequency, and photonic devices, energy storage and conversion systems, environmental applications and water treatment, smart materials and systems, and structural and thermal applications.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


