The effect of iron complexes−nitrilotriacetate in the bio−oxidation of sulphide minerals: Toxic metal(loid)s release and surface microstructure
IF 5.4
Q2 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
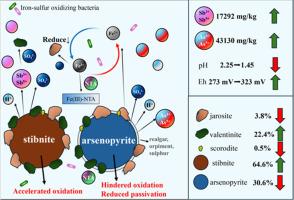
Mineral exploitation and processing lead to the production of a large amount of stibnite−containing waste, which are the main sources of toxic metal(loid)s pollution in soil and water ecosystems. Bio−oxidation constitutes an essential driving force for pollution formation. However, the release of Sb in the association of stibnite and arsenopyrite in the presence of nitrilotriacetic acid (NTA) is still unclear. In this study, stibnite, stibnite−arsenopyrite and stibnite−arsenopyrite−NTA were designed to study the release characteristics of antimony (Sb). And the microstructure of elements in the reaction microinterface was analyzed by high−precision spectral characterization such as synchrotron radiation X−ray diffraction (SRXRD) and X−ray photoelectron spectroscopy (XPS). The results showed that the cell density of the microbial community in the three groups did not change much in the early stage, and began to rise and gradually stabilized in the middle and late stage of the reaction, indicating that the biological reaction basically stopped. Arsenopyrite promotes the release of antimony from stibnite, and the addition of NTA further enhanced the release of Sb and arsenic (As), increasing 17,292 and 43,130 mg/kg. However, the concentration of Fe decreased but progressively increased due to the NTA added. Meanwhile, the existence of NTA decreased leachate pH from 2.25 to 1.45 and increased Eh from 273 mV to 323 mV. The presence of NTA reduced the FeAsS in the microinterface of minerals to 30.6 %. Meanwhile, Fe2(SO4)3 and FeAsO4·2H2O decreased to 3.8 % and 0.5 %, Sb2O5 and As(Ⅲ)−O increased to 22.4 % and 21.9 %. The abovementioned facts indicated that the complexation of NTA with Fe (Ⅲ) inhibits the formation of passivates such as jarosite and scorodite, thus promoting the bio−oxidation of arsenopyrite and stibnite. The new insight of stibnite bio−oxidation contributed to the understanding of sulfide oxidation mechanism and helped to put forward the control measures of pollution source in mining area.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of hazardous materials advances
Environmental Engineering
CiteScore
4.80
自引率
0.00%
发文量
0
审稿时长
50 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


