Design and optimization of encapsulated sensor materials with diverse binding sites for efficient cyanide ion detection
IF 4.3
2区 化学
Q1 SPECTROSCOPY
Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy
Pub Date : 2024-11-28
DOI:10.1016/j.saa.2024.125512
引用次数: 0
Abstract
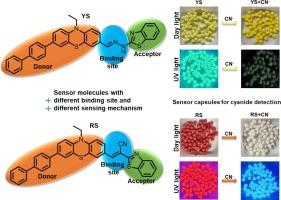
Developing colorimetric and fluorimetric sensors with a new design strategy incorporates the same electron donor and acceptor units by changing the binding site by expecting different mechanisms. The sensors YS and RS have the D-π-A concept, having phenothiazine as an electron donor and benzothiazole as an electron acceptor for sensing cyanide ions in various spectral techniques. Both the sensors showed an efficient color change with cyanide ion in day light and UV light, which was confirmed by UV–vis and Fluorescence spectral analysis. The mechanism of sensing cyanide ion by the sensor YS via hydrogen bond formation followed by deprotonation and RS via nucleophilic addition reaction was confirmed with a 1H NMR, FT-IR and HRMS spectral studies. The detection limit was found to be 1.36 μM and 0.78 μM by UV–vis, 0.13 nM and 0.39 nM by fluorescence technique are for sensors YS and RS, which are significantly lower than the WHO criterion of 1.9 μM for cyanide ions in water used for drinking. Furthermore, the real-world application showed that the sensors could quantitatively identify the quantity of cyanide ion present in different types of water samples. Besides, the fabricated test strips make the sensors easy to utilize for detecting CN− in the field without the need for complicated devices. Also, the developed sensor-encapsulated Polysulfone (PSF) capsule kit effectively senses cyanide ion in water.
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
8.40
自引率
11.40%
发文量
1364
审稿时长
40 days
期刊介绍:
Spectrochimica Acta, Part A: Molecular and Biomolecular Spectroscopy (SAA) is an interdisciplinary journal which spans from basic to applied aspects of optical spectroscopy in chemistry, medicine, biology, and materials science.
The journal publishes original scientific papers that feature high-quality spectroscopic data and analysis. From the broad range of optical spectroscopies, the emphasis is on electronic, vibrational or rotational spectra of molecules, rather than on spectroscopy based on magnetic moments.
Criteria for publication in SAA are novelty, uniqueness, and outstanding quality. Routine applications of spectroscopic techniques and computational methods are not appropriate.
Topics of particular interest of Spectrochimica Acta Part A include, but are not limited to:
Spectroscopy and dynamics of bioanalytical, biomedical, environmental, and atmospheric sciences,
Novel experimental techniques or instrumentation for molecular spectroscopy,
Novel theoretical and computational methods,
Novel applications in photochemistry and photobiology,
Novel interpretational approaches as well as advances in data analysis based on electronic or vibrational spectroscopy.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


