Enhanced chemical stability and H+/V4+ selectivity of microporous sulfonated polyimide via a triptycene-based crosslinker
IF 8.1
2区 工程技术
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
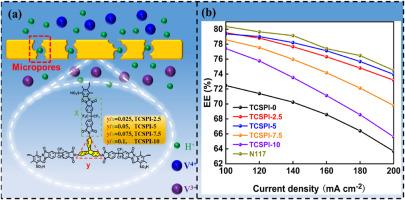
Long durability of sulfonated polyimide in vanadium redox flow battery (VRFB) is urgently required to be solved. Herein, we synthesize a triptycene-based crosslinker and use it as chemical crosslinking point to modify a linear sulfonated polyimide for promoting its antioxidative stability. The novel triptycene-based cross-linked sulfonated polyimide (TCSPI-X) membranes featuring covalently crosslinked network display lower water uptake and swelling ratio than the commercial perfluorinated ionomer membrane (Nafion 117) membrane. More importantly, unnoticeable proton conductivity loss is appeared. We speculate this is because of the covalently crosslinking structure provides stable proton transportation channels, and the formation of micropores induced by rigid triptycene unit decrease proton migration resistance. In which, the TCSPI-5 (with 5 % molar triptycene unit) exhibit higher voltage efficiency as compared with the pristine membrane TCSPI-0. Combined with the excellent vanadium ions resistance, the TCSPI-5 reaches energy efficiency of 78 % at the current density of 140 mA cm−2. In addition, TCSPI-5 also shows high oxidation resistance even under strong acid and pentavalent vanadium ions (V5+) conditions. The above results suggest the potential of TCSPI-X membranes in VRFB application.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Power Sources
工程技术-电化学
CiteScore
16.40
自引率
6.50%
发文量
1249
审稿时长
36 days
期刊介绍:
The Journal of Power Sources is a publication catering to researchers and technologists interested in various aspects of the science, technology, and applications of electrochemical power sources. It covers original research and reviews on primary and secondary batteries, fuel cells, supercapacitors, and photo-electrochemical cells.
Topics considered include the research, development and applications of nanomaterials and novel componentry for these devices. Examples of applications of these electrochemical power sources include:
• Portable electronics
• Electric and Hybrid Electric Vehicles
• Uninterruptible Power Supply (UPS) systems
• Storage of renewable energy
• Satellites and deep space probes
• Boats and ships, drones and aircrafts
• Wearable energy storage systems
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


