Ethyl 2-butene phosphite as a film-forming additive for high voltage lithium-ion batteries
IF 8.1
2区 工程技术
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
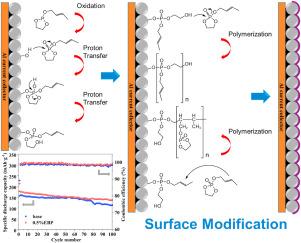
Increasing the charge cutoff voltage can significantly improve the capacity of lithium-ion batteries. However, the structural degradation of Ni-rich cathodes and high reactivity of electrolytes at the high-potential cathodes greatly affect the cycling stability. In this paper, a new type of cathode film-forming additive, ethyl 2-butene phosphite (EBP), is synthesized based on the molecular design of phosphite by increasing the functional group of carbon-carbon double bond and ring structure. Theoretical calculation shows that EBP has a higher HOMO level and can form a cathode electrolyte interphase (CEI) on the cathode electrode surface before the electrolyte in theory. The electrochemical performance of NCM622/Li half-cells is significantly enhanced by incorporating EBP into the electrolyte, achieving 72.52 % capacity retention over 100 cycles at 0.5C. Scanning Electron Microscope (SEM), X-Ray Diffraction (XRD), X-ray Photoelectron Spectroscopy (XPS) and Energy Dispersive Spectroscopy (EDS) are used to characterize the morphology of the anode and cathode, revealing that EBP forms a dense and complete CEI film on the surface of the LiNi0.6Co0.2Mn0.2O2 electrode. This film effectively blocks direct contact between the electrolyte and the cathode active material, prevents the dissolution of transition metals, improves interfacial stability, and consequently enhances the high-voltage cycling performance of the battery.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Power Sources
工程技术-电化学
CiteScore
16.40
自引率
6.50%
发文量
1249
审稿时长
36 days
期刊介绍:
The Journal of Power Sources is a publication catering to researchers and technologists interested in various aspects of the science, technology, and applications of electrochemical power sources. It covers original research and reviews on primary and secondary batteries, fuel cells, supercapacitors, and photo-electrochemical cells.
Topics considered include the research, development and applications of nanomaterials and novel componentry for these devices. Examples of applications of these electrochemical power sources include:
• Portable electronics
• Electric and Hybrid Electric Vehicles
• Uninterruptible Power Supply (UPS) systems
• Storage of renewable energy
• Satellites and deep space probes
• Boats and ships, drones and aircrafts
• Wearable energy storage systems
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


