Facilitating mechanisms of activity and SO2/H2O tolerance on ZrVOx catalysts for selective catalytic reduction of NOx removal with NH3
IF 13.3
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
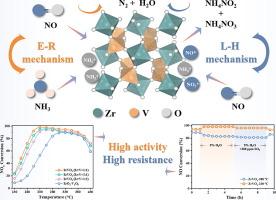
Sulfur oxides originated from high sulfur content in heavy fuel oil, which deleteriously impacting the catalysts during the NH3-SCR under low temperatures conditions. In this study, a ZrVOx catalyst with good low-temperature performance and resistance to SO2/H2O was synthesized via hydrothermal method for NH3-SCR. With the introduction of SO2 and H2O at 230 °C, the NOx conversion rate remained stable at 94 %. After characterization analysis, it was ascertained that the ZrVOx catalyst possessed a finely porous structure, expansive specific surface area, and strong low-temperature redox capability. Simultaneously, SO2 had minimal impact on the efficient adsorption of reactants., and deposited sulfate gradually decomposed with increasing temperature, mitigating the catalytic inhibition of SO2. The addition of H2O enhanced the concentration of surface active oxygen species (ROS) over the catalyst surface, increasing the surface hydroxyl groups and counteracted the negative impact of SO2 poisoning. Moreover, the in situ DRIFTs results confirmed that the catalytic reactions on ZrVOx primarily followed the Eley-Rideal (E-R) mechanism.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Engineering Journal
工程技术-工程:化工
CiteScore
21.70
自引率
9.30%
发文量
6781
审稿时长
2.4 months
期刊介绍:
The Chemical Engineering Journal is an international research journal that invites contributions of original and novel fundamental research. It aims to provide an international platform for presenting original fundamental research, interpretative reviews, and discussions on new developments in chemical engineering. The journal welcomes papers that describe novel theory and its practical application, as well as those that demonstrate the transfer of techniques from other disciplines. It also welcomes reports on carefully conducted experimental work that is soundly interpreted. The main focus of the journal is on original and rigorous research results that have broad significance. The Catalysis section within the Chemical Engineering Journal focuses specifically on Experimental and Theoretical studies in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. These studies have industrial impact on various sectors such as chemicals, energy, materials, foods, healthcare, and environmental protection.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


