Electroactive ferrocene-based ionic liquids: Transport and electrochemical properties of their acetonitrile solutions
IF 5.6
3区 材料科学
Q1 ELECTROCHEMISTRY
引用次数: 0
Abstract
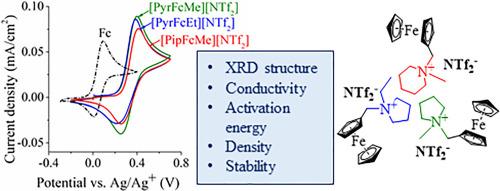
The increasing global demand for energy stimulates the development of advanced energy storage and conversion technologies. Modification of electrolyte with redox-active additives is a promising way to enhance the electrochemical performance of energy storage devices. In this work new electroactive ferrocene-based ionic liquids (Fc-ILs) (N-methyl-N-(ferrocenylmethyl)piperidinium bis(trifluoromethanesulfonyl)imide [PipFcMe][NTf2], N-methyl-N-(ferrocenylmethyl)pyrrolidinium bis(trifluoromethanesulfonyl)imide [PyrFcMe][NTf2], N-ethyl-N-(ferrocenylmethyl)pyrrolidinium bis(trifluoromethanesulfonyl)imide [PyrFcEt][NTf2]) were synthesized. The structure of the Fc-ILs was confirmed by NMR and XRD. [PipFcMe][NTf2] and [PyrFcMe][NTf2] demonstrated nearly similar crystal structures consisting of cation and anion columns, while alternating cation and anion layers are stacked in the [PyrFcEt][NTf2] crystal. The effects of the Fc-IL mole fraction and temperature on the transport properties of the acetonitrile solutions of the Fc-ILs have been established. The highest electrical conductivities of the [PipFcMe][NTf2], [PyrFcEt][NTf2] and [PyrFcMe][NTf2] solutions calculated using the Casteel-Amis equation were, respectively, 38.06 ± 0.63, 40.34 ± 0.93 and 41.09 ± 0.58 mS/cm at 348.15 K. The activation energies of conductivity were calculated using the Arrhenius and Vogel-Fulcher-Tamman approaches. The Fc-ILs demonstrated a high electrochemical stability range up to 4.67 V. The introduction of electron-withdrawing substituents into the cyclopentadienyl ring of Fc-ILs shifted the half-wave potential to the positive direction. The diffusion coefficients were calculated using the Randles–Ševčík equation for a quasi-reversible process.

电活性二茂铁离子液体:其乙腈溶液的输运和电化学性质
日益增长的全球能源需求刺激了先进能源存储和转换技术的发展。利用氧化还原活性添加剂对电解液进行改性是提高储能装置电化学性能的一种很有前途的方法。本文合成了新型电活性二茂铁离子液体(Fc-ILs) (n -甲基- n -(二茂铁甲基)吡啶双(三氟甲烷磺酰基)亚胺[PipFcMe][NTf2]、n -甲基- n -(二茂铁甲基)吡啶双(三氟甲烷磺酰基)亚胺[PyrFcMe][NTf2]、n -乙基- n -(二茂铁甲基)吡啶双(三氟甲烷磺酰基)亚胺[PyrFcEt][NTf2])。通过NMR和XRD对其结构进行了表征。[PipFcMe][NTf2]和[PyrFcMe][NTf2]表现出几乎相似的由正离子和阴离子柱组成的晶体结构,而[PyrFcEt][NTf2]晶体中的正离子和阴离子层是交替堆叠的。建立了Fc-IL摩尔分数和温度对Fc-IL乙腈溶液输运性质的影响。在348.15 K下,用ca钢- amis方程计算得到的[PipFcMe][NTf2]、[PyrFcEt][NTf2]和[PyrFcMe][NTf2]溶液的最高电导率分别为38.06±0.63、40.34±0.93和41.09±0.58 mS/cm。采用Arrhenius和Vogel-Fulcher-Tamman方法计算电导率的活化能。在高达4.67 V的电化学稳定范围内,Fc-ILs表现出很高的电化学稳定性。在fc - il的环戊二烯环中引入吸电子取代基,使半波电位向正方向移动。利用Randles -Ševčík方程计算准可逆过程的扩散系数。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Electrochimica Acta
工程技术-电化学
CiteScore
11.30
自引率
6.10%
发文量
1634
审稿时长
41 days
期刊介绍:
Electrochimica Acta is an international journal. It is intended for the publication of both original work and reviews in the field of electrochemistry. Electrochemistry should be interpreted to mean any of the research fields covered by the Divisions of the International Society of Electrochemistry listed below, as well as emerging scientific domains covered by ISE New Topics Committee.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


