Comprehensive Investigations of MUC1 O-Glycosylation Process Reveal Initial Site Preference by the Polypeptide GalNAc Transferases
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
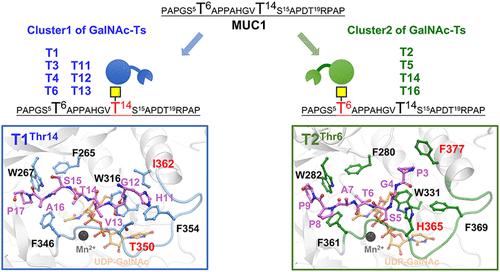
Tumor-associated MUC1 is coated with a high density of O-GalNAc glycans, which are initiated by a family of polypeptide N-acetyl-α-galactosaminyltransferases (GalNAc-Ts). However, the O-glycosylation process of MUC1 by each GalNAc-T isoform remains unclear. Here, we successfully obtained 14 human GalNAc-Ts with high catalytic activity based on a bacterial expression system. Employing MUC1-derived peptides as substrates, we systematically investigated the catalytic properties and site specificity of these GalNAc-Ts by chromatography and mass spectrometry, and found that they could be classified into two clusters. These two GalNAc-T clusters initially catalyze the threonine residue within GSTA or GVTS motifs, respectively, resulting in high O-glycosylation occupancy of both motifs. Moreover, molecular dynamics simulations and site-directed mutagenesis confirmed that the initial O-glycosite preference of GalNAc-Ts on MUC1 is controlled by two critical residues within the peptide-binding pocket. Swapping of the corresponding residues between two GalNAc-T clusters could exchange their initial O-glycosite preference. Quantum mechanics calculations further revealed the detailed catalytic mechanisms of GalNAc-Ts. Our work contributes to understanding the catalytic synthesis of multisite O-glycosylation of MUC1 by GalNAc-Ts, facilitating the development of O-glycosite-specific MUC1 vaccines.
MUC1 o -糖基化过程的综合研究揭示了多肽GalNAc转移酶的初始位点偏好
肿瘤相关MUC1被高密度的O-GalNAc聚糖包裹,该聚糖是由多肽n -乙酰-α-半乳糖氨基转移酶(GalNAc-Ts)家族启动的。然而,各GalNAc-T异构体MUC1的o -糖基化过程尚不清楚。在此,我们基于细菌表达系统成功获得了14个具有高催化活性的人GalNAc-Ts。以muc1衍生肽为底物,通过色谱和质谱分析系统地研究了这些GalNAc-Ts的催化性能和位点特异性,发现它们可以分为两个簇。这两个GalNAc-T簇最初分别催化GSTA或GVTS基序内的苏氨酸残基,导致两个基序的高o糖基化占用。此外,分子动力学模拟和定点诱变证实,GalNAc-Ts对MUC1的初始o -糖苷偏好是由肽结合口袋内的两个关键残基控制的。在两个GalNAc-T簇之间交换相应的残基可以交换它们初始的o -糖苷基偏好。量子力学计算进一步揭示了GalNAc-Ts的详细催化机理。我们的工作有助于理解GalNAc-Ts催化合成MUC1多位点o -糖基化,促进o -糖基化特异性MUC1疫苗的开发。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


