Purification of Nitrous Oxide via Thermal Decomposition with the Assistance of Methane: Mechanistic Study of By-Reactions
IF 3.9
3区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
Efficient and economical purification of nitrous oxide (N2O), one of the most abundant greenhouse gases, is urgently needed to prevent global warming, especially from exhaust emissions produced during adipic acid production. This study investigates the N2O thermal decomposition process via high-temperature incineration (800–1400 °C), as well as the effects of oxygen (O2) and methane (CH4) on deN2O efficiency and nitrogen selectivity. Under sufficient reaction conditions, deN2O efficiency reached 100% at ∼1000 °C. The introduction of CH4 was found to significantly enhance deN2O efficiency, with the addition of 5% CH4 resulting in complete N2O removal at <900 °C. Additionally, the influences of O2 and CH4 on the products nitric oxide and nitrogen dioxide (NO2) were analyzed via temperature-programmed reaction monitoring. Combined with the energy barriers obtained from density functional theory calculations, the reaction pathway network of N2O decomposition with and without CH4 was established. Moreover, the reaction rate equation for the crucial byproduct NO2 was derived from the elementary steps in the reaction network.
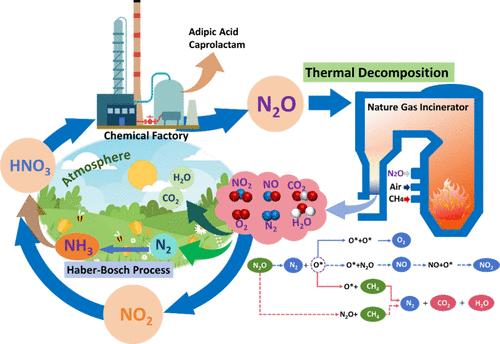
甲烷辅助热分解净化氧化亚氮:副反应机理研究
为了防止全球变暖,特别是己二酸生产过程中产生的废气排放,迫切需要高效、经济地净化一氧化二氮(N2O),这是最丰富的温室气体之一。本研究考察了高温焚烧(800-1400℃)N2O热分解过程,以及氧(O2)和甲烷(CH4)对deN2O效率和氮选择性的影响。在充分的反应条件下,在~ 1000°C时,deN2O效率达到100%。发现CH4的引入显著提高了deN2O的效率,添加5%的CH4可以在900°C下完全去除N2O。此外,通过程序升温反应监测分析了O2和CH4对产物一氧化氮和二氧化氮(NO2)的影响。结合密度泛函理论计算得到的能垒,建立了有CH4和无CH4分解N2O的反应途径网络。根据反应网络的基本步骤,推导出了关键副产物NO2的反应速率方程。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Industrial & Engineering Chemistry Research
工程技术-工程:化工
CiteScore
7.40
自引率
7.10%
发文量
1467
审稿时长
2.8 months
期刊介绍:
ndustrial & Engineering Chemistry, with variations in title and format, has been published since 1909 by the American Chemical Society. Industrial & Engineering Chemistry Research is a weekly publication that reports industrial and academic research in the broad fields of applied chemistry and chemical engineering with special focus on fundamentals, processes, and products.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


