Targeting histidinol-phosphate aminotransferase in Acinetobacter baumannii with salvianolic acid B: A structure-based approach to novel antibacterial strategies
IF 3.8
3区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
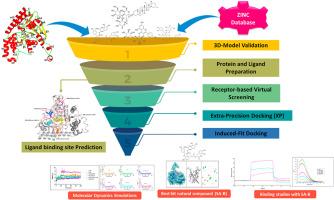
Undoubtedly, Acinetobacter baumannii is a major ESKAPE pathogen that poses a significant threat to public health, causing severe nosocomial infections with high mortality rates in healthcare settings. Due to the rapid development of antibiotic resistance, only a limited number of antibiotics remain effective against infections caused by multidrug-resistant (MDR) Acinetobacter baumannii. The discovery of new class of antibiotic molecules still lags behind the rate of growing worldwide burden of antimicrobial resistance (AMR). To expedite the discovery of new therapeutic molecules, we have focused on HisC from A. baumannii (AbHisC), a crucial enzyme involved in the seventh step of histidine biosynthesis. This pathway is absent in humans. We have employed the advanced computational techniques to target this promising drug target. AbHisC was cloned, overexpressed, and purified. Three distinct sets of libraries containing ∼60,000 natural compounds from ZINC database were screened against AbHisC using Schrödinger's glide module software. Based on the docking score and glide energy, top 25 hits were further subjected to induced fit (IF) docking. Top four out of the twenty five compounds from IF docking were subjected to 100ns molecular dynamics simulations, and it was observed that salvianolic acid B (SA-B) (a naturally occurring compound) complex with AbHisC, was found to be extremely stable. The glide energy and docking score of SA-B were −88.59 kcal/mol and −10.4 kcal/mol. SA-B was also found to quench the intrinsic fluorescence of tyrosine indicating its binding to the target. The dissociation constant calculated using Surface Plasmon Resonance was found to be 3.4x10−9 M. Based on these results we can conclude that SA-B can serve as the potential inhibitor of AbHisC that can further form the basis of structure based drug design against this deadly pathogen.
丹酚酸B靶向鲍曼不动杆菌组氨酸二醇-磷酸转氨酶:一种基于结构的新型抗菌策略
毫无疑问,鲍曼不动杆菌是一种主要的ESKAPE病原体,对公共卫生构成重大威胁,在卫生保健机构引起严重的医院感染,死亡率高。由于抗生素耐药性的迅速发展,只有有限数量的抗生素对多药耐药(MDR)鲍曼不动杆菌引起的感染有效。新一类抗生素分子的发现仍然落后于全球抗菌素耐药性负担的增长速度。为了加速新的治疗分子的发现,我们将重点放在鲍曼杆菌的HisC (AbHisC)上,这是一种参与组氨酸生物合成第七步的关键酶。这种途径在人类中不存在。我们采用先进的计算技术来定位这个有希望的药物靶点。克隆、过表达和纯化AbHisC。使用Schrödinger的glide模块软件,针对AbHisC对锌数据库中含有约60,000种天然化合物的三组不同的库进行筛选。根据对接得分和滑翔能量,对排名前25的命中目标进行诱导配合对接。对IF对接的25个化合物进行了100ns的分子动力学模拟,发现salvianolic acid B (SA-B)(一种天然存在的化合物)与AbHisC的配合物非常稳定。SA-B的滑行能量和对接分数分别为-88.59 kcal/mol和-10.4 kcal/mol。SA-B还能抑制酪氨酸的固有荧光,表明酪氨酸与靶标结合。利用表面等离子体共振计算的解离常数为3.4 × 10- 9m。基于这些结果,我们可以得出结论,SA-B可以作为AbHisC的潜在抑制剂,可以进一步形成基于结构的药物设计的基础,针对这种致命的病原体。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Archives of biochemistry and biophysics
生物-生化与分子生物学
CiteScore
7.40
自引率
0.00%
发文量
245
审稿时长
26 days
期刊介绍:
Archives of Biochemistry and Biophysics publishes quality original articles and reviews in the developing areas of biochemistry and biophysics.
Research Areas Include:
• Enzyme and protein structure, function, regulation. Folding, turnover, and post-translational processing
• Biological oxidations, free radical reactions, redox signaling, oxygenases, P450 reactions
• Signal transduction, receptors, membrane transport, intracellular signals. Cellular and integrated metabolism.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


