Design, Synthesis, and Biological Evaluation of [1,2,5]Oxadiazolo[3,4-b]pyridin-7-ol as Mitochondrial Uncouplers for the Treatment of Obesity and Metabolic Dysfunction-Associated Steatohepatitis
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
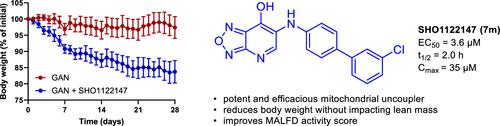
Mitochondrial uncouplers are small molecule protonophores that act to dissipate the proton motive force independent of adenosine triphosphate (ATP) synthase. Mitochondrial uncouplers such as BAM15 increase respiration and energy expenditure and have potential in treating a variety of metabolic diseases. In this study, we disclose the structure–activity relationship profile of 6-substituted [1,2,5]oxadiazolo[3,4-b]pyridin-7-ol derivatives of BAM15. Utilizing an oxygen consumption rate assay as a measure of increased cellular respiration, SHO1122147 (7m) displayed an EC50 of 3.6 μM in L6 myoblasts. Pharmacokinetic studies indicated a half-life of 2 h, Cmax of 35 μM, and no observed adverse effects at 1,000 mg kg–1 dose in mice. In a Gubra-Amylin (GAN) mouse model of MASH, SHO1122147 was efficacious in decreasing body weight and liver triglyceride levels at 200 mg kg–1 day–1 without changes in body temperature. These findings indicate the potential of utilizing novel [1,2,5]oxadiazolo[3,4-b]pyridin-7-ol mitochondrial uncouplers for treatment of fatty liver disease and obesity.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


