Unveiling the structural and functional perspectives of a bifunctional α-l-arabinofuranosidase/endo-β-1,4-xylanase (BoGH43_35) from Bacteroides ovatus
IF 3.8
3区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
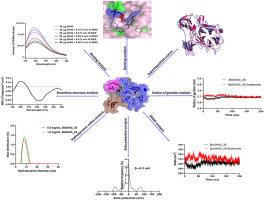
Arabinoxylan, a complex hemicellulose, can be degraded to its constituent sugars by concerted action of hemicellulases like α-l-arabinofuranosidase, endo-β-1,4-xylanase and xylosidase. In this study, a novel bifunctional α-l-arabinofuranosidase/endo-β-1,4-xylanase (BoGH43_35) of glycoside hydrolase family 43 subfamily 35 from Bacteroides ovatus was characterized by computational and experimental approaches. Sequence analysis identified Asp34 and Glu251 as the conserved catalytic residues. Structure analysis of BoGH43_35 disclosed 5-bladed β-propeller fold adopted by the N-terminal GH43 catalytic module followed by two independently folded carbohydrate-binding modules family 6 (CBM6A and CBM6B), displaying jellyroll type β-sandwich fold. Molecular Dynamics simulation of BoGH43_35 for 200 ns showed RMSD 0.35 nm, confirming structural stability and compactness of modeled structure. Molecular docking of BoGH43_35 with arabino-xylooligosaccharides and xylooligosaccharides by using AutoDock 4.2.7 demonstrated most favourable binding with arabinose (−5.01 kcal/mol) followed by arabinoxylobiose (−4.35 kcal/mol), xylotriose (−4.65 kcal/mol), xylotetraose (−4.18 kcal/mol) and xylobiose (−3.66 kcal/mol) showing affinity with both types of oligosaccharides. RMSD value of BoGH43_35-arabinose complex decreased to 0.28 nm upon MD simulation from 0.35 nm for only BoGH43_35, indicating stability of enzyme-substrate complex throughout the trajectory. The binding analysis of BoGH43_35 with wheat arabinoxylan by fluorescence spectroscopy gave Ka, 3.1 × 102 M−1, ΔG -14.2 kJ mole−1 and number of binding sites 2.2. Dynamic light scattering of BoGH43_35 showed hydrodynamic radius (Rh) of 4.0 nm, slightly higher than the radius of gyration (2.69 nm) from MD simulation. Additionally, zeta potential measurements (−9.3 mV at 0.5 mg/mL and −9.4 mV at 1.0 mg/mL) denoted its fair resistance towards aggregation in aqueous solution.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Archives of biochemistry and biophysics
生物-生化与分子生物学
CiteScore
7.40
自引率
0.00%
发文量
245
审稿时长
26 days
期刊介绍:
Archives of Biochemistry and Biophysics publishes quality original articles and reviews in the developing areas of biochemistry and biophysics.
Research Areas Include:
• Enzyme and protein structure, function, regulation. Folding, turnover, and post-translational processing
• Biological oxidations, free radical reactions, redox signaling, oxygenases, P450 reactions
• Signal transduction, receptors, membrane transport, intracellular signals. Cellular and integrated metabolism.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


