Optimizing extrusion processes and understanding conformational changes in itraconazole amorphous solid dispersions using in-line UV–Vis spectroscopy and QbD principles
IF 6.4
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
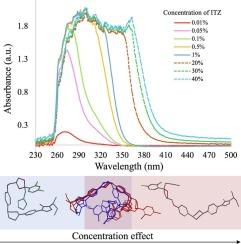
This paper presents a comprehensive investigation of the manufacturing of itraconazole (ITZ) amorphous solid dispersions (ASDs) with Kolllidon® VA64 (KVA64) using hot-melt extrusion (HME) and in-line process monitoring, employing a Quality by Design (QbD) approach. A sequential Design of Experiments (DoE) strategy was utilized to optimize the manufacturing process, with in-line UV–Vis spectroscopy providing real-time monitoring. The first DoE used a fractional factorial screening design to evaluate critical process parameters (CPPs), revealing that ITZ concentration had the most significant impact on the product quality attributes. The second DoE, employing a central composite design, explored the interactions between feed rate and screw speed, using torque and absorbance at 370 nm as responses to develop a design space. Validation studies confirmed process robustness across multiple days, with stable in-line UV–Vis spectra and consistent product quality using 30 % ITZ, 300 rpm, 150 °C and 7 g/min as the optimized process conditions. Theoretical and experimental analyses indicated that shifts in UV–Vis spectra at different ITZ concentrations were due to conformational changes in ITZ, which were confirmed through density functional theory (DFT) calculations and infrared spectroscopy. This work offers novel insights into the production and monitoring of ITZ-KVA64-ASDs, demonstrating that in-line UV–Vis spectroscopy is a powerful tool for real-time process monitoring and/or control.
利用紫外-可见光谱和QbD原理优化伊曲康唑非晶固体分散体的挤出工艺和了解其构象变化
本文采用设计质量(QbD)方法,采用热熔挤压(HME)和在线过程监控,对Kolllidon®VA64 (KVA64)制造伊曲康唑(ITZ)非晶固体分散体(ASDs)进行了全面研究。采用顺序实验设计(DoE)策略优化制造工艺,并利用在线紫外可见光谱提供实时监控。第一个DoE使用分数因子筛选设计来评估关键工艺参数(CPPs),结果表明ITZ浓度对产品质量属性的影响最为显著。第二个DoE采用了中心复合设计,探索了进料速率和螺杆转速之间的相互作用,利用扭矩和370 nm处的吸光度作为响应来开发设计空间。验证研究证实了该工艺在多天内的稳稳性,在30% ITZ、300 rpm、150°C和7 g/min的优化工艺条件下,具有稳定的在线UV-Vis光谱和一致的产品质量。理论和实验分析表明,在不同的ITZ浓度下,UV-Vis光谱的变化是由于ITZ的构象变化引起的,通过密度泛函理论(DFT)计算和红外光谱分析证实了这一点。这项工作为itz - kva64 - asd的生产和监测提供了新的见解,证明了在线UV-Vis光谱是实时过程监测和/或控制的强大工具。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

International Journal of Pharmaceutics: X
Pharmacology, Toxicology and Pharmaceutics-Pharmaceutical Science
CiteScore
6.60
自引率
0.00%
发文量
32
审稿时长
24 days
期刊介绍:
International Journal of Pharmaceutics: X offers authors with high-quality research who want to publish in a gold open access journal the opportunity to make their work immediately, permanently, and freely accessible.
International Journal of Pharmaceutics: X authors will pay an article publishing charge (APC), have a choice of license options, and retain copyright. Please check the APC here. The journal is indexed in SCOPUS, PUBMED, PMC and DOAJ.
The International Journal of Pharmaceutics is the second most cited journal in the "Pharmacy & Pharmacology" category out of 358 journals, being the true home for pharmaceutical scientists concerned with the physical, chemical and biological properties of devices and delivery systems for drugs, vaccines and biologicals, including their design, manufacture and evaluation. This includes evaluation of the properties of drugs, excipients such as surfactants and polymers and novel materials. The journal has special sections on pharmaceutical nanotechnology and personalized medicines, and publishes research papers, reviews, commentaries and letters to the editor as well as special issues.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


