Synthesis and red emission properties of indolo[3,2-b]carbazole derivatives modified by β-diketone boron difluoride
IF 3.3
3区 物理与天体物理
Q2 OPTICS
引用次数: 0
Abstract
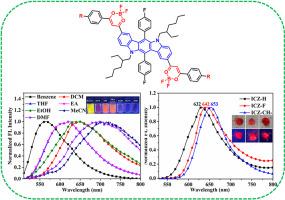
Organic fluorescent materials are extensively utilized in organic light-emitting diodes, biological imaging, fluorescent probes and other fields due to their easily controlled photophysical properties and high photostability. However, due to the constraints of energy band theory, compared to blue and green fluorescent materials, most organic red fluorescent materials possess large conjugated structures and introduce push-pull groups to regulate the distribution of electron clouds, which typically exhibit low fluorescent quantum efficiency. To investigate the regulatory mechanism of molecular spatial structure on the photophysical properties of red fluorescent materials, three derivatives of indolo[3,2-b]carbazole modified with β-diketone boron difluoride groups (named ICZ-H, ICZ-F and ICZ-CH3) were designed and synthesized. A systematic study was conducted on the photophysical properties in organic solvents and aggregated states, and the relationship between their spatial structure and properties was explored in detail. The UV–Vis absorption and fluorescent emission spectra in various organic solvents revealed that all three compounds exhibited strong absorption peaks near 490 nm, with maximum molar absorption coefficient exceeded 6.0 × 104 L/(mol cm)−1. The fluorescent intensities decreased with increasing solvent polarity, accompanied by a red-shifted in the fluorescent peak position. In the meantime, density functional theory calculations were utilized to visualize the process of intramolecular charge transfer. The aggregation fluorescent behaviors in a mixed solvents of tetrahydrofuran and water showed that the fluorescent intensity initially decreased and then increased, suggesting that all three indolo[3,2-b]carbazole derivatives retained the fluorescent emission property during the aggregation process. The fluorescent emission spectra in the solid state showed that the fluorescent peaks of all three compounds were all over 630 nm, which belonged to typical red fluorescent materials. The study on the structure-properties relationship of this material provides an experimental basis and theoretical guidance for the design of red-emitting fluorescent materials with excellent photophysical properties.
β-二酮二氟化硼修饰吲哚[3,2-b]咔唑衍生物的合成及红发射性质
有机荧光材料因其易于控制的光物理性质和光稳定性高而广泛应用于有机发光二极管、生物成像、荧光探针等领域。然而,由于能带理论的限制,与蓝色和绿色荧光材料相比,大多数有机红色荧光材料具有较大的共轭结构,并引入推拉基团来调节电子云的分布,其荧光量子效率通常较低。为研究分子空间结构对红色荧光材料光物理性能的调控机制,设计合成了三种以β-二酮二氟化硼基修饰的吲哚[3,2-b]咔唑衍生物(ICZ-H、ICZ-F和ICZ-CH3)。系统研究了其在有机溶剂和聚集态下的光物理性质,详细探讨了其空间结构与性质的关系。在各种有机溶剂中的紫外可见吸收光谱和荧光发射光谱表明,这3种化合物在490 nm附近都有很强的吸收峰,最大摩尔吸收系数超过6.0 × 104 L/(mol cm)−1。荧光强度随溶剂极性的增加而降低,荧光峰位置出现红移。同时,利用密度泛函理论计算可视化了分子内电荷转移的过程。在四氢呋喃和水的混合溶剂中的聚集荧光行为显示,荧光强度先降低后增加,这表明三种吲哚[3,2-b]咔唑衍生物在聚集过程中都保留了荧光发射特性。固态荧光发射光谱显示,三种化合物的荧光峰均在630 nm以上,属于典型的红色荧光材料。该材料的结构-性能关系的研究为设计具有优异光物理性能的红色荧光材料提供了实验依据和理论指导。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Luminescence
物理-光学
CiteScore
6.70
自引率
13.90%
发文量
850
审稿时长
3.8 months
期刊介绍:
The purpose of the Journal of Luminescence is to provide a means of communication between scientists in different disciplines who share a common interest in the electronic excited states of molecular, ionic and covalent systems, whether crystalline, amorphous, or liquid.
We invite original papers and reviews on such subjects as: exciton and polariton dynamics, dynamics of localized excited states, energy and charge transport in ordered and disordered systems, radiative and non-radiative recombination, relaxation processes, vibronic interactions in electronic excited states, photochemistry in condensed systems, excited state resonance, double resonance, spin dynamics, selective excitation spectroscopy, hole burning, coherent processes in excited states, (e.g. coherent optical transients, photon echoes, transient gratings), multiphoton processes, optical bistability, photochromism, and new techniques for the study of excited states. This list is not intended to be exhaustive. Papers in the traditional areas of optical spectroscopy (absorption, MCD, luminescence, Raman scattering) are welcome. Papers on applications (phosphors, scintillators, electro- and cathodo-luminescence, radiography, bioimaging, solar energy, energy conversion, etc.) are also welcome if they present results of scientific, rather than only technological interest. However, papers containing purely theoretical results, not related to phenomena in the excited states, as well as papers using luminescence spectroscopy to perform routine analytical chemistry or biochemistry procedures, are outside the scope of the journal. Some exceptions will be possible at the discretion of the editors.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


