Improving Na3V2(PO4)2F3 half-cell performance with NaBF4-enhanced sodium difluoro(oxalato)borate electrolyte
IF 14.9
1区 化学
Q1 Energy
引用次数: 0
Abstract
The global shift towards low-carbon energy storage has increased interest in sodium-ion batteries (SIBs) as a safer, cost-effective alternative to lithium-ion batteries. However, the commercial viability has been limited by compatibility issues between high-energy-density cathode materials, such as Na3V2(PO4)2F3 (NVPF), and high-voltage electrolytes. Addressing the challenges, H-NaODFB (comprising 93.91% NaODFB and 5.85% NaBF4) electrolyte significantly improves the electrochemical performance and stability of NVPF cathodes. Na/NVPF half-cells using H-NaODFB electrolyte retained 92.4% capacity after 900 cycles, while Na/Na symmetric cells demonstrated a cycle life exceeding 600 h at 0.5 mA cm−2. The superior performance is attributed to improved Na+ (de)intercalation reversibility, lower interfacial impedance (619.8 vs. 10,650.0 Ω), and faster reaction kinetics compared to NaODFB alone. Advanced time of flight-secondary ion mass spectrometry (TOF-SIMS), X-ray photoelectron spectroscopy (XPS) and aberration corrected transmission electron microscope (AC-TEM), combined with first-principles calculations, revealed that NaBF4 in the H-NaODFB electrolyte plays a critical role in forming a stable cathode electrolyte interphase (CEI). The CEI consists of an initial inorganic and organic layer, followed by a fluoroborate layer, and finally a stable organic–inorganic polymeric layer, enhancing electrode stability and preventing over-oxidation. These findings provide valuable insights for designing high-performance electrolytes for SIBs.
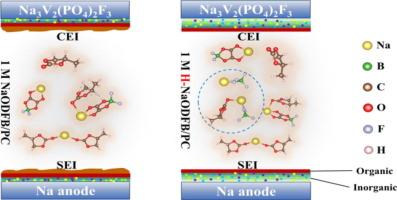
用nabf4增强的二氟硼酸钠电解质改善Na3V2(PO4)2F3半电池性能
全球向低碳能源存储的转变增加了人们对钠离子电池(sib)的兴趣,钠离子电池是锂离子电池的一种更安全、更经济的替代品。然而,商业可行性受到高能量密度阴极材料(如Na3V2(PO4)2F3 (NVPF))与高压电解质之间兼容性问题的限制。针对这一挑战,H-NaODFB(由93.91% NaODFB和5.85% NaBF4组成)电解质显著提高了NVPF阴极的电化学性能和稳定性。使用h - naodfb电解质的Na/NVPF半电池在900次循环后仍保持92.4%的容量,而Na/Na对称电池在0.5 mA cm−2下的循环寿命超过600小时。与NaODFB相比,其优越的性能归功于Na+ (de)嵌入可逆性的改善,更低的界面阻抗(619.8 vs. 10,650.0 Ω)和更快的反应动力学。先进的飞行时间离子质谱(TOF-SIMS)、x射线光电子能谱(XPS)和像差校正透射电镜(AC-TEM)结合第一线原理计算,揭示了H-NaODFB电解质中的NaBF4在形成稳定的阴极电解质间相(CEI)中起着关键作用。CEI由初始的无机和有机层组成,其次是氟硼酸盐层,最后是稳定的有机-无机聚合物层,增强了电极的稳定性并防止过氧化。这些发现为设计高性能sib电解质提供了有价值的见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Energy Chemistry
CHEMISTRY, APPLIED-CHEMISTRY, PHYSICAL
CiteScore
19.10
自引率
8.40%
发文量
3631
审稿时长
15 days
期刊介绍:
The Journal of Energy Chemistry, the official publication of Science Press and the Dalian Institute of Chemical Physics, Chinese Academy of Sciences, serves as a platform for reporting creative research and innovative applications in energy chemistry. It mainly reports on creative researches and innovative applications of chemical conversions of fossil energy, carbon dioxide, electrochemical energy and hydrogen energy, as well as the conversions of biomass and solar energy related with chemical issues to promote academic exchanges in the field of energy chemistry and to accelerate the exploration, research and development of energy science and technologies.
This journal focuses on original research papers covering various topics within energy chemistry worldwide, including:
Optimized utilization of fossil energy
Hydrogen energy
Conversion and storage of electrochemical energy
Capture, storage, and chemical conversion of carbon dioxide
Materials and nanotechnologies for energy conversion and storage
Chemistry in biomass conversion
Chemistry in the utilization of solar energy
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


