Insights into reaction mechanisms: Water’s role in enhancing in-situ hydrogen production from methane conversion in sandstone
IF 13.1
1区 化学
Q1 Energy
引用次数: 0
Abstract
In-situ conversion of subsurface hydrocarbons via electromagnetic (EM) heating has emerged as a promising technology for producing carbon-zero and affordable hydrogen (H2) directly from natural gas reservoirs. However, the reaction pathways and role of water as an additional hydrogen donor in EM-assisted methane-to-hydrogen (CH4-to-H2) conversion are poorly understood. Herein, we employ a combination of lab-scale EM-heating experiments and reaction modeling analyses to unravel reaction pathways and elucidate water’s role in enhancing hydrogen production. The labelled hydrogen isotope of deuterium oxide (D2O) is used to trace the sources of hydrogen. The results show that water significantly boosts hydrogen yield via coke gasification at around 400 °C and steam methane reforming (SMR) reaction at over 600 °C in the presence of sandstone. Water-gas shift reaction exhibits a minor impact on this enhancement. Reaction mechanism analyses reveal that the involvement of water can initiate auto-catalytic loop reactions with methane, which not only generates extra hydrogen but also produces OH radicals that enhance the reactants’ reactivity. This work provides crucial insights into the reaction mechanisms involved in water-carbon-methane interactions and underscores water’s potential as a hydrogen donor for in-situ hydrogen production from natural gas reservoirs. It also addresses the challenges related to carbon deposition and in-situ catalyst regeneration during EM heating, thus derisking this technology and laying a foundation for future pilots.
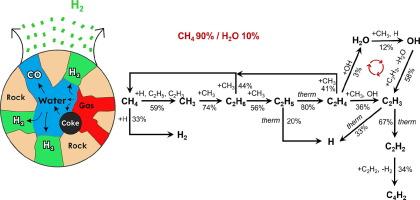
对反应机制的洞察:水在提高砂岩中甲烷转化的原位产氢中的作用
通过电磁(EM)加热对地下碳氢化合物进行原位转化,已经成为一项有前途的技术,可以直接从天然气储层中生产零碳和价格合理的氢(H2)。然而,在em辅助甲烷-氢(CH4-to-H2)转化过程中,水作为额外的氢供体的反应途径和作用尚不清楚。本文采用实验室规模的电磁加热实验和反应模型分析相结合的方法来揭示反应途径,并阐明水在促进氢生产中的作用。氧化氘(D2O)的标记氢同位素用于追踪氢的来源。结果表明,在砂岩存在下,在400℃左右的焦炭气化和600℃以上的蒸汽甲烷重整(SMR)反应中,水显著提高了氢的产率。水气转换反应对这种增强的影响较小。反应机理分析表明,水的参与可以引发与甲烷的自催化环反应,不仅产生多余的氢,还会产生OH自由基,提高反应物的反应活性。这项工作为水-碳-甲烷相互作用的反应机制提供了重要的见解,并强调了水作为天然气储层原位制氢的氢供体的潜力。它还解决了与EM加热过程中碳沉积和原位催化剂再生相关的挑战,从而降低了该技术的风险,为未来的试点奠定了基础。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Energy Chemistry
CHEMISTRY, APPLIED-CHEMISTRY, PHYSICAL
CiteScore
19.10
自引率
8.40%
发文量
3631
审稿时长
15 days
期刊介绍:
The Journal of Energy Chemistry, the official publication of Science Press and the Dalian Institute of Chemical Physics, Chinese Academy of Sciences, serves as a platform for reporting creative research and innovative applications in energy chemistry. It mainly reports on creative researches and innovative applications of chemical conversions of fossil energy, carbon dioxide, electrochemical energy and hydrogen energy, as well as the conversions of biomass and solar energy related with chemical issues to promote academic exchanges in the field of energy chemistry and to accelerate the exploration, research and development of energy science and technologies.
This journal focuses on original research papers covering various topics within energy chemistry worldwide, including:
Optimized utilization of fossil energy
Hydrogen energy
Conversion and storage of electrochemical energy
Capture, storage, and chemical conversion of carbon dioxide
Materials and nanotechnologies for energy conversion and storage
Chemistry in biomass conversion
Chemistry in the utilization of solar energy
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


