Single-atomic iron synergistic atom-cluster induce remote enhancement toward oxygen reduction reaction
IF 13.1
1区 化学
Q1 Energy
引用次数: 0
Abstract
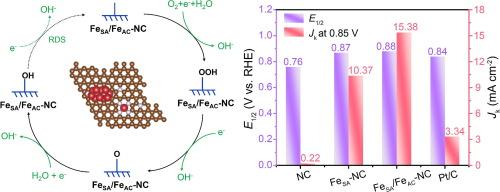
The oxygen reduction reaction (ORR) could be effectively regulated by adjusting electron configurations and optimizing chemical bonds. Herein, we have achieved the modulation of electron distribution in Fe single atomic (FeSA) sites through Fe atomic clusters (FeAC) via a confined pyrolysis approach, thereby enhancing their intrinsic ORR activity. X-ray absorption spectroscopy has confirmed that the presence of iron atomic clusters could influence the electron distribution at Fe-N4 sites. The FeSA/FeAC-NC catalyst exhibits a half-wave potential of 0.88 V, surpassing the individual FeSA-NC structure. Through electronic structure analysis, it could be seen that iron atom clusters can affect Fe-N4 sites through long-range effects, and then effectively lower reaction barriers and enhance the reaction kinetics at Fe-N4 sites. The synthetic approach might pave the way for constructing highly active catalysts with tunable atomic structures, representing an effective and universal technique for electron modulation in M-N-C systems. This work provides enlightenment for the exploration of more efficient single-atom electrocatalysts and the optimization of the performance of atomic electrocatalysts. Furthermore, a zinc-air battery assembled using it on their cathode deliver a high peak power density (205.7 mW cm−2) and a high-specific capacity of 807.5 mA h g−1. This study offers a fresh approach to effectively enhance the synergistic interaction of between Fe single atom and Fe atomic clusters for improving ORR activity and energy storage.
单原子铁协同原子团簇诱导氧还原反应的远程增强
通过调整电子构型和优化化学键可以有效地调控氧还原反应。在此,我们通过限制性热解方法通过铁原子团簇(FeAC)实现了铁单原子(FeSA)位点的电子分布调制,从而增强了它们的本征ORR活性。x射线吸收光谱证实了铁原子团簇的存在会影响Fe-N4位点的电子分布。FeSA/FeAC-NC催化剂的半波电位为0.88 V,超过了单个FeSA- nc结构。通过电子结构分析可知,铁原子团簇可以通过远程效应影响Fe-N4位点,从而有效降低Fe-N4位点的反应势垒,提高反应动力学。该合成方法可能为构建具有可调原子结构的高活性催化剂铺平道路,代表了M-N-C系统中电子调制的有效和通用技术。这项工作为探索更高效的单原子电催化剂和优化原子电催化剂的性能提供了启示。此外,在阴极上使用它组装的锌空气电池提供了高峰值功率密度(205.7 mW cm - 2)和807.5 mA h g - 1的高比容量。该研究为有效增强Fe单原子与Fe原子团簇之间的协同相互作用以提高ORR活性和能量储存提供了新的途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Energy Chemistry
CHEMISTRY, APPLIED-CHEMISTRY, PHYSICAL
CiteScore
19.10
自引率
8.40%
发文量
3631
审稿时长
15 days
期刊介绍:
The Journal of Energy Chemistry, the official publication of Science Press and the Dalian Institute of Chemical Physics, Chinese Academy of Sciences, serves as a platform for reporting creative research and innovative applications in energy chemistry. It mainly reports on creative researches and innovative applications of chemical conversions of fossil energy, carbon dioxide, electrochemical energy and hydrogen energy, as well as the conversions of biomass and solar energy related with chemical issues to promote academic exchanges in the field of energy chemistry and to accelerate the exploration, research and development of energy science and technologies.
This journal focuses on original research papers covering various topics within energy chemistry worldwide, including:
Optimized utilization of fossil energy
Hydrogen energy
Conversion and storage of electrochemical energy
Capture, storage, and chemical conversion of carbon dioxide
Materials and nanotechnologies for energy conversion and storage
Chemistry in biomass conversion
Chemistry in the utilization of solar energy
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


