Pretargeted alpha therapy in MUC16-positive high-grade serous ovarian cancer
IF 3.6
4区 医学
Q1 RADIOLOGY, NUCLEAR MEDICINE & MEDICAL IMAGING
引用次数: 0
Abstract
Background
Peritoneal metastasis with micrometastatic cell clusters is a common feature of advanced ovarian cancer. Targeted alpha therapy (TAT) is an attractive approach for treating micrometastatic diseases as alpha particles release enormous amounts of energy within a short distance. A pretargeting approach — leveraging the inverse-electron-demand Diels-Alder reaction between tetrazines (Tz) and trans-cyclooctene (TCO) — can minimize off-target toxicity related to TAT, often associated with full-length antibodies. We hypothesized that a pretargeting strategy could effectively treat high-grade serous (HGS) ovarian tumors while minimizing toxicity.
Methods
We utilized the humanized antibody, AR9.6, labeled with actinium-225 (225Ac). AR9.6 targets fully glycosylated and hypoglycosylated isoforms of MUC16. For biodistribution and radioimmunotherapy studies, AR9.6-TCO was injected into OVCAR3-bearing mice 72 h before administering [225Ac]Ac-mcp-PEG8-Tz, e.g. using a 1,2,4,5-tetrazine conjugated to the macropa chelator via a polyethylene glycol (PEG) linker.
Results
Biodistribution data revealed that the pretargeting approach achieved substantial tumor uptake. Cerenkov luminescence imaging confirmed successful in vivo pretargeting during TAT studies. Compared to the control groups, TAT with AR9.6-TCO and [225Ac]Ac-mcp-PEG8-Tz significantly suppressed tumor growth and improved overall survival in OVCAR3 tumor-bearing mice. Renal and ovarian pathology compatible with toxicity was observed in mice in addition to transient hematologic toxicity.
Conclusion
We confirmed that pretargeting with AR9.6-TCO and [225Ac]Ac-mcp-PEG8-Tz has durable antitumor effects in high MUC16-expressing tumors. These findings demonstrate great potential for using pretargeting in combination with TAT for the treatment of ovarian cancer.
Classification
Biological Sciences; Applied Biological Sciences.
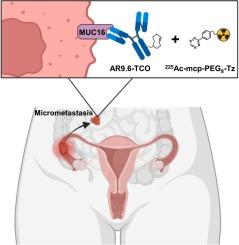
muc16阳性的高级别浆液性卵巢癌的预靶向治疗
伴微转移细胞团的腹膜转移是晚期卵巢癌的共同特征。靶向α治疗(TAT)是治疗微转移性疾病的一种有吸引力的方法,因为α粒子在短距离内释放大量能量。预靶向方法-利用四嗪(Tz)和反式环烯(TCO)之间的逆电按需diols - alder反应-可以最大限度地减少与TAT相关的脱靶毒性,通常与全长抗体相关。我们假设预先靶向策略可以有效治疗高级别浆液性卵巢肿瘤,同时最小化毒性。方法采用actinium-225 (225Ac)标记的人源抗体AR9.6。AR9.6靶向MUC16的完全糖基化和低糖基化亚型。为了进行生物分布和放射免疫治疗研究,在给药[225Ac]Ac-mcp-PEG8-Tz前72小时,将AR9.6-TCO注射到携带ovcar3的小鼠体内,例如,通过聚乙二醇(PEG)连接剂将1,2,4,5-四嗪偶联到巨噬细胞螯合剂上。结果生物分布数据显示,预靶向方法实现了大量的肿瘤摄取。切伦科夫发光成像证实了TAT研究中成功的体内预靶向。与对照组相比,AR9.6-TCO和[225Ac]Ac-mcp-PEG8-Tz的TAT可显著抑制OVCAR3肿瘤小鼠的肿瘤生长,提高总生存率。除了短暂的血液学毒性外,在小鼠中还观察到与毒性相容的肾脏和卵巢病理。结论AR9.6-TCO和[225Ac]Ac-mcp-PEG8-Tz对muc16高表达肿瘤具有持久的抗肿瘤作用。这些发现表明,使用预先靶向联合TAT治疗卵巢癌具有巨大的潜力。ClassificationBiological科学;应用生物科学。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nuclear medicine and biology
医学-核医学
CiteScore
6.00
自引率
9.70%
发文量
479
审稿时长
51 days
期刊介绍:
Nuclear Medicine and Biology publishes original research addressing all aspects of radiopharmaceutical science: synthesis, in vitro and ex vivo studies, in vivo biodistribution by dissection or imaging, radiopharmacology, radiopharmacy, and translational clinical studies of new targeted radiotracers. The importance of the target to an unmet clinical need should be the first consideration. If the synthesis of a new radiopharmaceutical is submitted without in vitro or in vivo data, then the uniqueness of the chemistry must be emphasized.
These multidisciplinary studies should validate the mechanism of localization whether the probe is based on binding to a receptor, enzyme, tumor antigen, or another well-defined target. The studies should be aimed at evaluating how the chemical and radiopharmaceutical properties affect pharmacokinetics, pharmacodynamics, or therapeutic efficacy. Ideally, the study would address the sensitivity of the probe to changes in disease or treatment, although studies validating mechanism alone are acceptable. Radiopharmacy practice, addressing the issues of preparation, automation, quality control, dispensing, and regulations applicable to qualification and administration of radiopharmaceuticals to humans, is an important aspect of the developmental process, but only if the study has a significant impact on the field.
Contributions on the subject of therapeutic radiopharmaceuticals also are appropriate provided that the specificity of labeled compound localization and therapeutic effect have been addressed.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


