In-situ confinement growth of FeNi alloy within B/N co-doped carbon nanotubes as efficient electrocatalyst for water splitting
IF 3.9
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Developing low-cost and efficient catalysts for hydrogen evolution reaction (HER) and oxygen evolution reaction (OER) still remains a challenge in water electrolysis. Herein, FeNi alloy encapsulated within boron and nitrogen co-doped carbon (FeNi@BNC) nanotubes are synthesized through a simple one-step pyrolysis method using FeCl3·6H2O, NiCl2·6H2O, H3BO3, urea and PEG-2000 as precursors. The BNC nanotubes are quite requisite for dispersing and stabilizing FeNi alloy nanoparticles (NPs) during pyrolysis. Benefiting from the synergistic catalytic effect of Fe and Ni, as well as the confinement effect of BNC nanotubes, such FeNi@BNC catalyst demonstrates impressive activities for both HER and OER, much superior to pristine Fe@BNC and Ni@BNC. Notably, the overpotentials needed to achieve a current density of 10 mA·cm−2 are just 230 mV for HER and 280 mV for OER. Moreover, the FeNi@BNC catalyst demonstrates significant stability, showing no noticeable degradation during potentiostatic electrolysis or repeated CV tests. Furthermore, FeNi@BNC exhibits remarkable activity for overall water splitting, requiring cell voltages of just 1.24 V and 1.60 V vs. RHE to achieve current densities of 10 mA·cm−2 and 20 mA·cm−2, respectively. This study introduces a novel strategy for developing bifunctional electrocatalysts with high-efficiency water splitting performance.
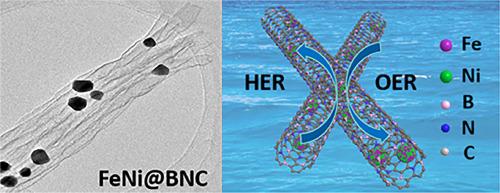
B/N共掺杂碳纳米管中FeNi合金的原位约束生长作为水裂解的高效电催化剂
开发低成本、高效的析氢反应(HER)和析氧反应(OER)催化剂仍然是水电解领域面临的挑战。本文以FeCl3·6H2O、NiCl2·6H2O、H3BO3、尿素和PEG-2000为前驱体,采用简单的一步热解方法,在硼氮共掺杂碳纳米管(FeNi@BNC)内包封FeNi合金。BNC纳米管是FeNi合金纳米颗粒在热解过程中分散和稳定所必需的。得益于Fe和Ni的协同催化效应,以及BNC纳米管的约束效应,这种FeNi@BNC催化剂在HER和OER方面都表现出令人瞩目的活性,远远优于原始的Fe@BNC和Ni@BNC。值得注意的是,实现10 mA·cm−2电流密度所需的过电位在HER和OER中仅为230 mV和280 mV。此外,FeNi@BNC催化剂表现出显著的稳定性,在恒电位电解或重复CV测试中没有明显的降解。此外,FeNi@BNC具有显著的整体水分解活性,相对于RHE,仅需1.24 V和1.60 V的电池电压即可分别达到10 mA·cm - 2和20 mA·cm - 2的电流密度。本研究介绍了一种开发具有高效水分解性能的双功能电催化剂的新策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Molecular Catalysis
Chemical Engineering-Process Chemistry and Technology
CiteScore
6.90
自引率
10.90%
发文量
700
审稿时长
40 days
期刊介绍:
Molecular Catalysis publishes full papers that are original, rigorous, and scholarly contributions examining the molecular and atomic aspects of catalytic activation and reaction mechanisms. The fields covered are:
Heterogeneous catalysis including immobilized molecular catalysts
Homogeneous catalysis including organocatalysis, organometallic catalysis and biocatalysis
Photo- and electrochemistry
Theoretical aspects of catalysis analyzed by computational methods
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


