Hydrotalcite-derived well-dispersed and thermally stable cobalt nanoparticle catalyst for ammonia decomposition
IF 4.9
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Ammonia is a carbon-free hydrogen carrier, and development of non-noble metal catalyst to decompose ammonia into hydrogen is desirable for practical applications. However, the metal catalyst is challenged by the sintering of metal particles under high-temperature reaction conditions. In this study, a series of Li-, Al-, and Co-containing hydrotalcite-like compounds (HTlc) were synthesized by co-precipitation and used as precursors to prepare well-dispersed and thermally stable Co nanoparticle catalysts for ammonia decomposition. The obtained precursors and catalysts were characterized by means of X-ray powder diffraction (XRD), temperature-programmed reduction (H2-TPR), X-ray photoelectron spectroscopy (XPS), high-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM), and so on. All of the precursors formed hydrotalcite-like phase, which consisted of Li–Al–(Co) HTlc and/or Co–Al HTlc dependent on the Co content. Upon calcination at 500 °C, HTlc decomposed into an Al-substituted Co3O4 spinel oxide, as confirmed by two distinctly separated reduction steps in H2-TPR. Following reduction at 700 °C, well-dispersed Co metal nanoparticles with an average particle size of ∼9.2–12.4 nm were obtained. It was suggested that the incorporation of Al3+ into Co3O4 led to a strong interaction between cobalt and aluminum, which suppressed the crystal growth of Co3O4 and the sintering of Co metal during the thermal treatments, resulting in good Co dispersion. The optimal LiAlCo(1.5) catalyst showed superior activity than that prepared by impregnation method, giving almost complete conversion of ammonia at 575 °C under a space velocity of 5,000 mL gcat–1 h–1. More importantly, this catalyst maintained stable activity at 625 °C for 100 h, exhibiting high stability and sintering resistance. The good catalytic performance was attributed to the high Co metal dispersion and strong metal–support interaction benefiting from the uniform distribution of cobalt in the HTlc precursor. These results demonstrate the applicability of HTlc to the preparation of metal catalysts with improved dispersion and thermal stability.
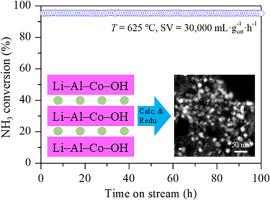
水滑石衍生的分散良好、热稳定的氨分解钴纳米颗粒催化剂
氨是一种无碳氢载体,因此开发非贵金属催化剂将氨分解成氢气是实际应用的理想选择。然而,金属催化剂在高温反应条件下面临金属颗粒烧结的挑战。本研究采用共沉淀法合成了一系列含锂、铝和钴的类水滑石化合物 (HTlc),并以此为前驱体制备了分散良好、热稳定的 Co 纳米粒子催化剂,用于氨分解。通过 X 射线粉末衍射 (XRD)、温度编程还原 (H2-TPR)、X 射线光电子能谱 (XPS)、高角度环形暗场扫描透射电子显微镜 (HAADF-STEM) 等方法对得到的前驱体和催化剂进行了表征。所有前驱体都形成了类似水滑石的相,根据 Co 含量的不同,由 Li-Al-(Co) HTlc 和/或 Co-Al HTlc 组成。在 500 °C 煅烧时,HTlc 分解成 Al 取代的 Co3O4 尖晶石氧化物,这一点在 H2-TPR 中两个明显分开的还原步骤中得到了证实。在 700 °C 下还原后,得到了分散良好的 Co 金属纳米颗粒,平均粒径为 9.2-12.4 nm。研究表明,在 Co3O4 中加入 Al3+ 会导致钴和铝之间产生强烈的相互作用,从而在热处理过程中抑制了 Co3O4 的晶体生长和 Co 金属的烧结,使 Co 得到良好的分散。最佳的 LiAlCo(1.5) 催化剂比浸渍法制备的催化剂具有更高的活性,在 575 °C 下,空间速度为 5,000 mL gcat-1 h-1 时,氨几乎完全转化。更重要的是,这种催化剂能在 625 °C 下保持 100 小时的稳定活性,表现出很高的稳定性和抗烧结性。良好的催化性能归功于 HTlc 前驱体中钴的均匀分布所带来的高钴金属分散性和强金属-支撑相互作用。这些结果表明 HTlc 适用于制备具有更好分散性和热稳定性的金属催化剂。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Molecular Catalysis
Chemical Engineering-Process Chemistry and Technology
CiteScore
6.90
自引率
10.90%
发文量
700
审稿时长
40 days
期刊介绍:
Molecular Catalysis publishes full papers that are original, rigorous, and scholarly contributions examining the molecular and atomic aspects of catalytic activation and reaction mechanisms. The fields covered are:
Heterogeneous catalysis including immobilized molecular catalysts
Homogeneous catalysis including organocatalysis, organometallic catalysis and biocatalysis
Photo- and electrochemistry
Theoretical aspects of catalysis analyzed by computational methods
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


