Molecular oxygen uptake behavior of crystalline porous complex metal oxide based on ε-Keggin polyoxometalate unit framework
IF 4.9
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
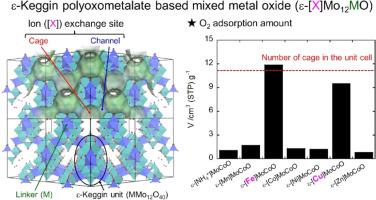
ε-Keggin polyoxometalate based complex metal oxides are composed of the structural arrangement of ε-Keggin [Mo12O40], which connects with octahedral linker, {MO6}, to form a microporous structure that localizes counter cations X similar to FAU-type zeolites. In the present study, O2 adsorption properties of these materials (ε-[X]MoMO) are investigated. ε-[X]MoCoO with X = Fe and Cu showed significant O2 adsorption in an irreversible manner and the adsorption amount was saturated when 1.0 of O2 was adsorbed in the unit cell. The ε-Keggin unit of these materials were oxidized after the O2 adsorption at room temperature, resulting in the shrinkage of their lattice. Based on the characterization results and adsorption behavior, we suggest the O2 adsorption behavior as follows; O2 can freely access the whole ε-Keggin unit in ε-[Fe or Cu]MoCoO through the micropores involving redox between O2 and the ε-Keggin unit. The oxidation of this unit reduces the lattice size, which slows down the migration of O2 and results in the quasi-equilibrium adsorption near the surface. The selection of M element in ε-[Fe]MoMO was also crucial for the O2 adsorption.
基于ε-Keggin多金属氧酸盐单元框架的晶体多孔复合金属氧化物的分子吸氧行为
ε-Keggin多金属氧酸盐基络合金属氧化物由ε-Keggin [Mo12O40]的结构排列组成,ε-Keggin与八面体连接剂{MO6}连接,形成微孔结构,类似于fu型沸石,定位反阳离子X。本文研究了ε-[X]MoMO材料对O2的吸附性能。X = Fe和Cu的ε-[X]MoCoO对O2有明显的不可逆吸附,当吸附量为1.0时吸附量达到饱和。这些材料的ε-Keggin单元在室温O2吸附后被氧化,导致其晶格收缩。根据表征结果和吸附行为,我们认为O2的吸附行为如下:O2可以通过O2与ε-[Fe或Cu]MoCoO中ε- keggin单元之间的氧化还原微孔自由进入ε-[Fe或Cu]MoCoO中的整个ε- keggin单元。该单元的氧化降低了晶格尺寸,减缓了O2的迁移,导致表面附近的准平衡吸附。ε-[Fe]MoMO中M元素的选择对O2的吸附也至关重要。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Molecular Catalysis
Chemical Engineering-Process Chemistry and Technology
CiteScore
6.90
自引率
10.90%
发文量
700
审稿时长
40 days
期刊介绍:
Molecular Catalysis publishes full papers that are original, rigorous, and scholarly contributions examining the molecular and atomic aspects of catalytic activation and reaction mechanisms. The fields covered are:
Heterogeneous catalysis including immobilized molecular catalysts
Homogeneous catalysis including organocatalysis, organometallic catalysis and biocatalysis
Photo- and electrochemistry
Theoretical aspects of catalysis analyzed by computational methods
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


