Theoretical insights into H2S desulfurization: Catalysis by binuclear cobalt phthalocyanine
IF 4.9
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
The desulfurization process of H2S catalyzed by binuclear cobalt phthalocyanine (bi-CoPc) has been explored theoretically. With the support of density functional theory (DFT) calculation, the desulfurization mechanism consists of active species and sulfur chain growing. Firstly, the active species has been identified, where the specific electron transfer occurs from HS−, traverses through the phthalocyanine ring, and culminates at O2. This process leads to the formation of Co(I)-Co(I) complexes. Secondly, the sulfur chain growing to S2 on bi-CoPc and detaching in the form of S22− have been considered as the optimal path. Furthermore, a feasible and efficient bi-CoPc-based catalysts with larger π-conjugated system (naphthyl or anthracyl) has been proposed, seem to positively impact electron storage capacity and desulfurization efficiency.
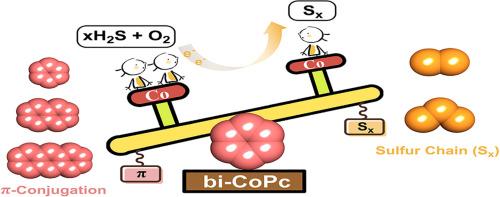
硫化氢脱硫的理论见解:双核钴酞菁催化
对双核酞菁钴(bi-CoPc)催化H2S脱硫过程进行了理论探讨。在密度泛函理论(DFT)计算的支持下,脱硫机理由活性物质和硫链生长组成。首先,已经确定了活性物质,其中特定的电子转移发生在HS−,穿过酞菁环,并在O2处达到高潮。这一过程导致Co(I)-Co(I)配合物的形成。其次,硫链在bi-CoPc上生长为S2,并以S22 -的形式分离为最优路径。此外,还提出了一种可行且高效的双氯化碳基催化剂,其π共轭体系(萘基或蒽基)较大,似乎对电子存储容量和脱硫效率有积极影响。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Molecular Catalysis
Chemical Engineering-Process Chemistry and Technology
CiteScore
6.90
自引率
10.90%
发文量
700
审稿时长
40 days
期刊介绍:
Molecular Catalysis publishes full papers that are original, rigorous, and scholarly contributions examining the molecular and atomic aspects of catalytic activation and reaction mechanisms. The fields covered are:
Heterogeneous catalysis including immobilized molecular catalysts
Homogeneous catalysis including organocatalysis, organometallic catalysis and biocatalysis
Photo- and electrochemistry
Theoretical aspects of catalysis analyzed by computational methods
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


