In vitro activation of recombinant pro-protein-glutaminases from Bacteroides helcogenes and Flavobacterium sp.
IF 3.9
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Protein-glutaminases (PGs) are extracellular enzymes and catalyze the deamidation of protein-bound glutamine residues in other proteins. This often increases the respective protein's solubility. Therefore, PGs are interesting for the food industry since protein-rich foods are trending. Microbial PGs are intracellularly produced in their inactive zymogenic pre-pro-form and subsequently activated after the secretion process, whereby the pre-pro-peptide sequence is cleaved off extracellularly by an endogenous peptidase. However, a former study showed that a recombinantly produced pro-PG from Bacteroides helcogenes (PGB) was surprisingly active, although the pro-peptide was still attached. In this study, the specific in vitro cleavage of the PGB pro-peptide by HRV-3C peptidase was investigated and led to a 16-fold activity increase compared to the non-cleaved pro-PGB. Alternatively, trypsin was used for activation (17.5-fold activity increase) and mass spectrometry analysis revealed that the pro-peptide was indeed cleaved in proximity to the putative native cleavage site. In addition, another formerly unknown PG from Flavobacterium sp. 316 (PGF) was recombinantly produced as pro-PGF in E. coli, partially purified, and biochemically characterized. Pro-PGF was almost inactive, but was activated similarly to pro-PGB through in vitro pro-peptide hydrolysis with trypsin. The mature PGF showed maximal specific activity of 123.7 nkat mg−1 at 35 °C and pH 9.
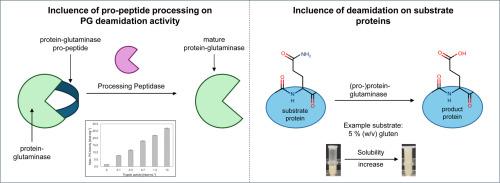
helcogenes拟杆菌和黄杆菌重组原蛋白-谷氨酰胺酶的体外活化。
蛋白质-谷氨酰胺酶(pg)是细胞外酶,催化其他蛋白质中结合的谷氨酰胺残基的脱酰胺。这通常会增加相应蛋白质的溶解度。因此,pg对于食品行业来说是有趣的,因为富含蛋白质的食品是趋势。微生物PGs是在细胞内以其无活性的酶原前形式产生的,随后在分泌过程中被激活,其中前前肽序列被内源性肽酶在细胞外切割。然而,先前的一项研究表明,重组产自helcogenes拟杆菌(Bacteroides helcogenes, PGB)的前pg具有惊人的活性,尽管前肽仍然附着在细菌上。在这项研究中,研究了HRV-3C肽酶对PGB前肽的体外特异性切割,其活性比未切割的PGB前肽提高了16倍。另外,胰蛋白酶被用于激活(活性增加17.5倍),质谱分析显示前肽确实在假定的天然裂解位点附近被裂解。此外,我们在大肠杆菌中重组生产了另一种来自Flavobacterium sp. 316 (PGF)的原PGF,并对其进行了部分纯化和生化表征。Pro-PGF几乎没有活性,但通过胰蛋白酶体外前肽水解,其活性与pro-PGB相似。成熟的PGF在35°C和pH为9时的最大比活性为123.7 nkat mg−1。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Molecular Catalysis
Chemical Engineering-Process Chemistry and Technology
CiteScore
6.90
自引率
10.90%
发文量
700
审稿时长
40 days
期刊介绍:
Molecular Catalysis publishes full papers that are original, rigorous, and scholarly contributions examining the molecular and atomic aspects of catalytic activation and reaction mechanisms. The fields covered are:
Heterogeneous catalysis including immobilized molecular catalysts
Homogeneous catalysis including organocatalysis, organometallic catalysis and biocatalysis
Photo- and electrochemistry
Theoretical aspects of catalysis analyzed by computational methods
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


