Qingke Pingchuan granules alleviate airway inflammation in COPD exacerbation by inhibiting neutrophil extracellular traps in mice
IF 6.7
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
Background
Chronic obstructive pulmonary disease (COPD) imposes a significant global health and socioeconomic burden. Exacerbations of COPD (ECOPD), characterized by heightened airway inflammation and mucus hypersecretion, adversely affect patient health and accelerate disease progression. Qingke Pingchuan (QKPC) granules, a formulation from Traditional Chinese Medicine initially prescribed for acute bronchitis, have shown unexplored potential in ECOPD management, with mechanisms of action yet to be clarified.
Purpose
This study investigates the therapeutic effects of QKPC in a mouse model of ECOPD, focusing on underlying molecular mechanisms.
Methods
COPD was induced in mice through chronic cigarette smoke (CS) exposure, followed by intratracheal administration of Pseudomonas aeruginosa lipopolysaccharide (LPS) to trigger exacerbation, after which mice were treated with QKPC granules. Major compounds in QKPC were identified via UHPLC-QE-MS, and high-throughput RNA sequencing of lung tissue samples identified differentially expressed genes. Transcriptomic data were integrated with network pharmacology analysis to pinpoint potential pathways, bioactive compounds, and target genes through which QKPC might attenuate ECOPD. Molecular docking, protein-small molecule binding assays, and in vitro analyses further validated interactions between key compounds and target genes, shedding light on plausible signaling pathways.
Results
QKPC treatment led to significant reductions in airway leukocyte infiltration and goblet cell metaplasia in CS- and LPS-exposed mice, accompanied by decreased levels of inflammatory cytokines (IL-6, IL-1β, CXCL1, and TNF-α) and mucin MUC5AC in bronchoalveolar lavage fluid. The integrative transcriptomic and network pharmacology analysis identified the neutrophil extracellular trap (NET) formation pathway as a key mechanism underlying QKPC's protective effect against ECOPD. In vitro assays demonstrated that epigallocatechin-3-gallate (EGCG) and quercetin, two important bioactive compounds in QKPC, significantly inhibited NETosis induced by cigarette smoke extract (CSE) plus LPS in human neutrophils. The two compounds were found to interact directly with the reactive oxidative species (ROS)-generating enzyme NOX2 and its regulatory subunit p47phox. Subsequent in vitro studies further confirmed EGCG and quercetin's capacity to reduce ROS production and downregulate NOX2 and p47phox protein levels in neutrophils stimulated with CSE and LPS. Additionally, in vivo studies confirmed QKPC's efficacy in reducing NET formation, oxidative stress, and NOX2/p47phox protein expression in the lung tissue of ECOPD mice.
Conclusion
These findings suggest that QKPC granules alleviate airway inflammation in ECOPD, potentially through inhibition of pulmonary NET formation via the NOX2/p47phox-ROS pathway, underscoring their potential therapeutic application for ECOPD management in clinical settings.
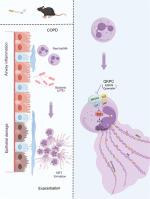
清咳平喘颗粒通过抑制小鼠中性粒细胞胞外陷阱减轻COPD加重期气道炎症
慢性阻塞性肺疾病(COPD)对全球健康和社会经济造成了重大负担。COPD (ECOPD)的恶化以气道炎症加剧和粘液分泌过多为特征,对患者健康产生不利影响并加速疾病进展。清咳平喘颗粒是一种最初用于治疗急性支气管炎的中药制剂,在ECOPD治疗中显示出未开发的潜力,其作用机制尚不清楚。目的:探讨芪芩素对ECOPD小鼠模型的治疗作用,并探讨其分子机制。方法通过慢性香烟烟雾(CS)诱导小鼠scopd,然后气管内给药铜绿假单胞菌脂多糖(LPS)引发加重,然后给药芪芩颗粒剂。通过UHPLC-QE-MS对QKPC中的主要化合物进行鉴定,并对肺组织样品进行高通量RNA测序,鉴定差异表达基因。转录组学数据与网络药理学分析相结合,以确定QKPC可能减轻ECOPD的潜在途径、生物活性化合物和靶基因。分子对接、蛋白-小分子结合试验和体外分析进一步验证了关键化合物与靶基因之间的相互作用,揭示了可能的信号通路。结果qkpc可显著降低CS和lps暴露小鼠气道白细胞浸润和杯状细胞化生,并降低支气管肺泡灌洗液中炎症因子(IL-6、IL-1β、CXCL1、TNF-α)和粘蛋白MUC5AC水平。综合转录组学和网络药理学分析发现,中性粒细胞胞外陷阱(NET)形成途径是QKPC对ECOPD保护作用的关键机制。体外实验表明,表没食子儿茶素-3-没食子酸酯(EGCG)和槲皮素是QKPC中两种重要的生物活性化合物,可显著抑制香烟烟雾提取物(CSE)加LPS诱导的中性粒细胞NETosis。这两种化合物与活性氧生成酶NOX2及其调控亚基p47phox直接相互作用。随后的体外研究进一步证实了EGCG和槲皮素在CSE和LPS刺激的中性粒细胞中减少ROS生成和下调NOX2和p47phox蛋白水平的能力。此外,在体内研究证实了QKPC在ECOPD小鼠肺组织中具有降低NET形成、氧化应激和NOX2/p47phox蛋白表达的作用。结论芪芪颗粒剂可能通过NOX2/p47phox-ROS通路抑制肺NET的形成,从而减轻ECOPD患者的气道炎症,强调其在ECOPD临床治疗中的潜在应用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Phytomedicine
医学-药学
CiteScore
10.30
自引率
5.10%
发文量
670
审稿时长
91 days
期刊介绍:
Phytomedicine is a therapy-oriented journal that publishes innovative studies on the efficacy, safety, quality, and mechanisms of action of specified plant extracts, phytopharmaceuticals, and their isolated constituents. This includes clinical, pharmacological, pharmacokinetic, and toxicological studies of herbal medicinal products, preparations, and purified compounds with defined and consistent quality, ensuring reproducible pharmacological activity. Founded in 1994, Phytomedicine aims to focus and stimulate research in this field and establish internationally accepted scientific standards for pharmacological studies, proof of clinical efficacy, and safety of phytomedicines.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


